Review Article - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Various Promising Biological Effects of Cranberry Extract: A Review
Raghad Riyadh Khali*, Eman Tareq Mohammed and Yasser Fakri MustafaRaghad Riyadh Khali, Department of Pharmaceutical Chemistry, University of Mosul, Mosul, Iraq, Email: raghadalbarhawi@uomosul.edu.iq
Received: 04-Aug-2021 Accepted Date: Aug 18, 2021 ; Published: 25-Aug-2021
Abstract
Cranberry is a well-known natural occurring product that has been used for centuries in traditional medicine to promote urinary tract health. Besides that, Cranberry has shown promising biomedicine activity in the prevention and treatment of many human disorders. The presence of polyphenolic entities such as proanthocyanidins, anthocyanins, flavonols, and other chemo-constituents is commonly linked to the positive effects of this natural product on human health. Cranberry and its extract were developed and marketed as nutraceutical supplements, and their antioxidant, antibacterial, anti-inflammatory, and anticancer properties allow them to be used for managing a variety of ailments, including microbial infections, metabolic syndrome elements, and cancer. The focus of this paper is on some of the recent studies which explore the potential pharmacological activities of Cranberry extract. Ultimately, this study concluded that cranberry extract can offer a feasible and prospective phytochemical option for treating many disorders affecting human health.
Keywords
Cranberry •Cranberry extract •Antimicrobial •Antioxidant •Anti-inflammatory
Introduction
Nature, predominately through plants, has always been an inexhaustible source of bioactive compounds. In many different cultures, phytotherapy has had a well-known effect on the treatment and prevention of various diseases [1]. According to the World Health Organization (WHO), about eighty percent of the world's population, primarily in developing countries, relies on conventional plant-derived medicines for primary health care, while plant products continue to play a significant, albeit indirect role in the health care systems of the developed countries [2]. Better compatibility with the human body and relatively fewer side effects of botanical-based medicine, in comparison with synthetic compounds, may shift the global trend of synthetic drugs, in the past few decades, to herbal medicines, that intimates a return to nature to treat different ailments [3-5].
North American Cranberry (Vaccinium macrocarpon), which belongs to the Ericaceae family is a woody, low-growing, vining perennial plant native to northeastern North America, extending from eastern Canada to North Carolina in the United States. This edible red fruit is one of the economically significant North American fruits [6], while Vaccinium oxycoccus is the European variety, which is grown in portions of central Europe, Germany, and Finland. Vaccinium oxycoccus is a smaller fruit with slightly different acid profiles and anthocyanin than the North American variant [7]. Native Americans employed the Vaccinium macrocarpon as a food source and natural deterrent against bladder and kidney disorders. Furthermore, during ocean trips, American sailors used Cranberries as an antiscorbutic agent [6,8].
The promising health potential of V. macrocarpon, which received the most attention for its advantageous effects on human health dating back to the 17th century, led within the last few decades to the popularity of Cranberry's dietary supplements as an alternative and convenient dietary source of Cranberry phytochemicals. These supplements profess to be manufactured from concentrated Cranberry extracts, freeze-dried Cranberry powders, or Cranberry phenolic isolate products and advertised for their high equivalence values to the fresh fruit and a variety of putative health advantages, including urinary tract health support [9-12].
Chemo-constituents of cranberry and its cultivars
Cranberries are a varied and abundant source of phytochemicals. More than one hundred fifty phytochemicals have been discovered and studied in Cranberry and its cultivars so far. However, more ingredients can be discovered as analytical techniques developed [13].
Flavonoids seem to be the most predominant chemo-constituent phenotype. Also, other chemo-constituents have been recognized, such as Anthocyanins, which give Cranberries their bright red color; proanthocyanidins, which protect against urinary tract infections; flavonols, the secondary yellowish pigments; terpene; and pectins; While the sour and astringent flavor of Cranberries is due to catechins, organic acids, and resveratrol [13].
Anthocyanins
Anthocyanins are arguably the most investigated phytoconstituent and are responsible for the fruit's characteristic red color. Anthocyanins are found mainly in the colored bodies of the exocarp layer of the fruit's pericarp, and they increase as the fruit matures [14-16]. Whole, raw Cranberry fruits contain substantial albeit inconsistent quantities of anthocyanins, ranging from 13.6 to 140 mg/100 g depending primarily on the cultivar, place of growth, fruit size, climatic, genetic, environmental, and other factors [13,16].
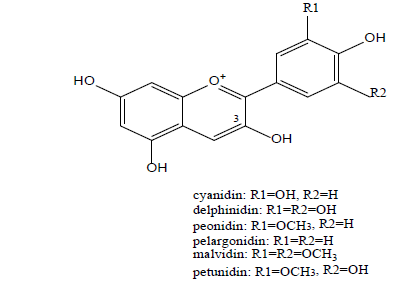
Chemically, anthocyanins consisting of an anthocyanidin backbone attached to a single or multiple sugar moieties. V. macrocarpon is one of the rare foods that muster glycosides of six aglycones of the anthocyanidin family: petunidin, cyanidin, delphinidin, pelargonidin, peonidin, and malvidin (Figure 1). The prevalent anthocyanins are 3-O-arabinosides and 3-O-galactosides of peonidin and cyanidin; a total of thirteen anthocyanins, mainly 3-O-monoglycosides, have been discovered [7,17,18].
Viskelis et al. Investigated four American Cranberry cultivars and reported that the average composition of the anthocyanins in these cultivars was as the following: peonidin-3-galactoside (32.7% ± 1.2%), cyanidin-3- galactoside (20.5% ± 1.8%), cyanidin-3-arabinoside (19% ± 3.3%), peonidin-3-arabinoside (6.7% ± 1.2%) peonidin-3-glucoside (3.5% ± 1.3%), and cyanidin-3-glucoside (2.3% ± 0.3%) [19]. However, recent analyzes have revealed a difference in the anthocyanin content of different processed Cranberry products and supplements [20–22].
Flavonols
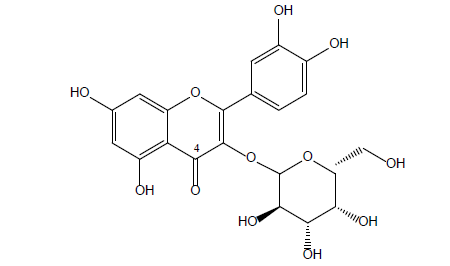
Flavonols are found in a multitude of Cranberry fruits, mostly in glycosylated forms of myricetin, quercetin, and to a lesser extent, kaempferol. Quercetin glycosides (mainly quercetin 3-O-galactoside) (Figure 2) forming about 75% of the Cranberry flavonols. A wide range of flavonols was detected, many of which are found in low concentrations, such as quercetin, myricetin, myricetin 3-O-arabinofuranoside, myricetin 3-O-galactoside, myricetin 3-O-xylopyranoside, myricetin 3-O-arabinopyranoside, and myricetin 3-O-rhamnoside [14,23–27].
Flavan-3-ols are significant metabolites of Cranberry. They possess the flavonoid scaffold as flavonols but without the C4 carbonyl group. They arise naturally in the plant as aglycons of epicatechin and catechin. Unlike other flavonoids, their good solubility allows them to stay in the vacuole with no need for glycosylation [7,13]. Flavonols and flavan-3-ols have received a lot of researchers' interest due to their supposed beneficial health properties [23].
Flavan-3-ols are significant metabolites of Cranberry. They possess the flavonoid scaffold as flavonols but without the C4 carbonyl group. They arise naturally in the plant as aglycons of epicatechin and catechin. Unlike other flavonoids, their good solubility allows them to stay in the vacuole with no need for glycosylation [7,13]. Flavonols and flavan-3-ols have received a lot of researchers' interest due to their supposed beneficial health properties [23].
Proanthocyanidins
One of the exceptional properties of Cranberries is their diverse group of proanthocyanidins (PACs) content, also referred to as non-hydrolysable condensed tannins,that exhibit several unique structural characteristics. The flavan-3-ols mostly epicatechin and catechin are the monomeric units used by the plant to assemble oligomeric or polymeric structures known as PACs.
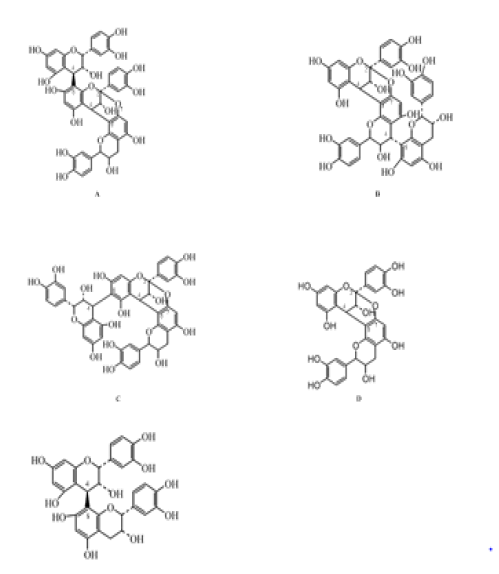
Although the mechanism by which this condensation occurs in the plant is not fully understood, two types of this chemical process have been identified. In the less commonly type named B, the condensation occurs via an inter-flavan bond between the flavan-3-ol units, where these monomeric units coupled primarily by bonds between C-4 and C-8, though connections between C-4 and C-6. On the other hand, type-A proanthocyanidins are characterized by an additional bond between C-2 and C-7 of the underlying flavan-3-ol units (Figure 3) [29-31].
Figure 3. Chemical backbones of the PAC trimers and dimers isolated from Cranberries. (A) epicatechin- (4-6)-epicatechin-(4-8,2-O-7)- epicatechin, (B) epicatechin-(4-8,2-O-7)-epicatechin- (4-8)-epicatechin, (C) epicatechin-(4-8)- epicatechin-(4-8,2-O-7)-epicatechin,(D) epicatechin-(4- 8,2-O-7)-epicatechin, (E) epicatechin- (4-8)-epicatechin (13).
The degree of PAC polymerization (DP) is determined by the source and processing of the fruit. PACs with DP equal to 23 and 26 have been detected in Cranberries using mass spectroscopy. These structural characteristics of PACs are responsible for their various and interesting bioactivities [29,32,33].
According to Gardana et al., the PAC content of different Cranberry cultivars ranging from 1471 mg/100 g-6563 mg/100 g of dry weight [34], while commercial extracts are standardized to 15% and 33% PAC. Because of this high content, Cranberry is considered as an expensive natural extract. Unfortunately, this has resulted in the adulteration of Cranberrybased products when inexpensive sources of PACs are employed and not disclosed on the label [13,34,35].
Other chemo-constituents
In the American version of the cranberry fruit, derivatives of the ursane type of triterpenoids, such as betulinic acid, oleanolic acid, and ursolic acid, were identified. In a study of six American Cranberry cultivars, Oszmia´nski et al. discovered that ursolic acid, the pentacyclic triterpene, was the most abundant triterpenoid (ranging from 50%-37%), followed by oleanolic acid (ranging from 28%-35%), and betulinic acid (ranged from 19%-28%) [36].
In addition, Cranberry contains phenolic acids, including hydroxycinnamic and hydroxybenzoic acid derivatives. The former is the most abundant. The foremost phenolic acids in Cranberry are caffeic acid, chlorogenic acid, ferulic acid, gallic acid, p-coumaric acid, protocatechuic acid, 3-hydroxybenzoic acid, 3-hydroxyphenylpropionic acid, 4-hydroxybenzoic acid, and 4-hydroxyphenylacetic [37].
Cranberry supplements may show ingredients profile that is somewhat different from that found in the other Cranberry products. Ursolic acid and oleanolic acid are found primarily in the fruit peel, which may not be used in supplements manufacturing. In most cases, there is no clear information on the label about the sources and forms used, whether whole fruit, juice, or extract. The reduced PAC content in most supplements could be due to the peel removal during the manufacturing process. The fruits are often squeezed or pressed during the Cranberries processing, and the residual pomace is dumped as a waste product. Furthermore, any fruit processing that requires extended exposure to light and heat has the potential to destroy the existing anthocyanin molecules [22,38].
Biomedical Activities
Anti-pathogenic potentials
Antiviral effect: With the rise in antiviral resistance and the emergence of new viral pathogens, there is an urgent need for antiviral medications that are commonly available, inexpensive and have few adverse effects. Many traditional medicines, which contain various plant metabolites, exhibited significant antiviral properties are now validated as new antivirals. Recently, studies have increasingly harangued the suitability of Cranberry extracts as promising antiviral agents [39,40].
Non-Dialyzable Materials (NDM) of high molecular weight isolated from Cranberry juice were reported to reduce influenza virus adhesion and infectivity; probably by preventing viral adsorption onto the cells [41]. Where Weiss et al. discovered that low NDM concentrations, which are twentyfold lower than those found in Cranberry juice, inhibits hemagglutination of RBCs induced by both influenza virus B and the A subtypes (H3N2 and H1N1). Pre-incubation of Madine-Darby Canine Kidney (MDCK) cells with NDM (250 g/ml) considerably reduced the infectivity of the influenza A and B subtypes, as evidenced by the lack of cytopathic effect on MDCK cells and hemagglutination activity in the medium of infected cells. Suggesting it hinders viral offspring generated by infected cells from adsorbing onto new cells [42].
Because Hemagglutinin (HA) and Neuraminidase (NA), the influenza virus surface glycoproteins, are crucial for the replication and infection of the virus, Oiknine-Djian et al. Examined the NDM's effect on neuraminidases, as these are the target of most marketed anti-influenza medications. They found that the enzymatic activity of NA of both influenza virus A and B strains in addition to that of Streptococcus pneumoniae can be inhibited by NDM. The NDM's anti-NA activity was found to be promising against different influenza virus strains and moderately active against Streptococcus pneumoniae NA. This finding is significant in light of the increasing prevalence of influenza isolates resistant to antiviral medicines, which has reached 90% in some areas. In addition to the therapeutic potential against both A and B influenza virus infections, this study suggested that cranberry constituents might also stymie the development of subsequent secondary bacterial complications [43].
In this context, V. macrocarpon extracts may give prospective chemicals capable of preventing viral attachment to the target cells basing on their known anti-adhesive actions against bacteria [44].
Oximacro, traditionally available Cranberry extract containing a high concentration of PACs type-A, has been shown to have a significant dosedependent antiviral effect against influenza A and B viruses, according to Luganini et al. Mechanistic experiments have demonstrated that the extract may inhibit the binding and penetration of influenza virus into target cells plus its virucidal properties. It was discovered that the extract may interact with the ectodomain of the viral hemagglutinin glycoprotein, implying interference with hemagglutinin activities and, as a result, loss of influenza virus particle infectivity. Fluorescence spectroscopy and in silico docking simulations confirmed the in-vitro findings, indicating that PAC-A2 (Dimeric catechin) is the predominant anti-influenza virus component among the several ingredients of this extract. The involvement of PAC-A2 in anti-influenza virus action was further verified when it was shown that it suppressed influenza virus reproduction. Overall, these findings point to Cranberry extracts as a promising candidate for developing innovative natural antiviral medicines to prevent influenza virus infections [45].
Hydrogen bonding, electrostatic interactions, van der Waals, as well as covalent bond formation, are expected to play a major role in the development of protein-PAC complexes. In this context, Terlizzi et al. found that the PACs type-A of Oximacro extract may protect against Herpes Simplex Type 1 (HSV-1) and Herpes Simplex Type 2 (HSV-2) infection through a similar biochemical process. This route involving changes in the envelope glycoproteins essential for entrance, such as gB and gD. The antiadhesive activity of this extract on HSV is attributable to direct contacts with the virion surface, as evidenced by interaction studies between PACs and either HSV particles or the ectodomain of pure gD protein.
Moreover, Cranberry extract may inhibit the in vitro replication of these DNA viruses. Even at acidic pH values (3.0- 4.0) and in the presence of 10% human serum proteins, this extract was maintained its anti-HSV activity, simulating the physiological features of the vagina as a prospective therapeutic location [46].
Overall, these findings point to Cranberry extract as a promising candidate for developing innovative natural antiviral medicines to prevent and treat RNA and DNA viruses' infections. The antiviral activity of Cranberry extract against human norovirus surrogates, Feline Calicivirus (FCV-F9), Murine Norovirus (MNV-1), and bacteriophage MS2 has been also investigated [47-49].
Based on the aforementioned studies, the proposed mechanism of the Cranberry extract could be associated with the direct binding between procyanidins and viral capsid proteins. This interaction can afford viral particle aggregation, which cause significant structural and morphological changes in the viral capsid and/or blocking of antigenic binding sites, resulting in reduced overall viral infectivity [50-52]. The integrity of the rotavirus capsid appears to be compromised by Cranberry's PACs. Direct viral testing indicates not only the absence of viral antigen but also a PAC-related inhibition or alteration of the rotavirus antigenic attachment determinants. These events appear to significantly lower the rotavirus infectivity [52-54].
Based on the findings of the studies involved in this review, Cranberry extract appears to have the potential as a natural antiviral remedy for treating and preventing foodborne and other virus infections. Also, the Covid-19 crisis highlighted the urgent need for improving the currently-available therapeutic facilities and exploring the antiviral potentials of various natural occurring products [40,55].
Antibacterial effect: Cranberry extract has been shown to have an antimicrobial activity against various pathogenic bacteria, including Staphylococcus aureus, E. coli, Helicobacter pylori, Campylobacter, and Salmonella. This would explain the apparent significance of the extract to prevent certain infectious diseases, such as Urinary Tract Infections (UTIs), stomach ulcers, and tooth decay [19].
UTI is one of the most prevalent bacterial illnesses in humans, with Escherichia coli being the most common cause. UTI affects up to 40%–50% of women at some point during their lives. The high frequency of UTIs, as well as the alarming growth in the antibiotic resistance among uropathogens, highlight the need for novel approaches to treat and prevent UTIs [56,57].
For too many years, Cranberry juice is being used as a remedy to treat and avert UTIs. Spanned to the present of some evidence that Cranberry juice can reduce the number of symptomatic urinary tract infections over one year, especially in women who have recurrent UTIs. These findings led the French Food Safety Authority to make the first-ever health claim on berry phenolics in 2004: A daily intake of 36 mg of Cranberry proanthocyanidins helps reduce the adherence of certain E. coli bacteria to the epithelium of the urinary tract [19,56].
Thereby, many studies have demonstrated the effectiveness of PACstandardized Cranberry supplements against bacterial adhesion and virulence in the urinary tract. Thus, these supplements can offer a means to cure and prevent UTIs [56,58]. Oral supplementation of a 120 mg capsule of Cranberry extract, standardized to 36 mg proanthocyanidins, for sixty days provided a prophylaxis in young, healthy subjects suffering from recurrent UTIs [59]. Furthermore, oral Cranberry supplementation reported to be effective in preventing UTI recurrence in healthy women [60].
Postmenopausal women, pregnant as well as lactating women, elderly men with benign prostatic hyperplasia, and children [61-64].
It is believed that the PACs, particularly type A phenotype, may act on the initial step of infection by inhibiting E. coli from the adherence to uroepithelial cells in a dose-dependent manner. PAC can bind to the E. coli and render bacteria non-adherent, probably by binding to fimbrial tips and compressing them, reducing adhesive forces and consequently influencing the first stage of infection. Also, PAC can decrease the number of fimbriae in E. coli in addition to compressing them. Because these mechanisms do not kill bacteria, there is a lower chance of resistant bacterial strains being selected. PAC derived from Cranberries may inhibit the multi-drug resistant E. coli strains from adhering to uroepithelial cells by 70% [29,58].
Another mechanism postulated is that quinic acid enhances the excretion of hippuric acid in the urine, which has an antibacterial effect, thus prevents the bacterial infection. Another explanation is that fructose inhibits fimbriated E. Coli from adhering to uroepithelial cells. While another theory proposed that Cranberry can affect the concentration of Tamm-Horsfall glycoproteins in urine, preventing E. coli from adherence to the human kidney [65,66].
Helicobacter pylori is a spiral-shaped Gram-negative bacteria that infect over half of the world's population. Chronic gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and gastric cancer are all thought to be caused by H. pylori infection in the stomach. It's difficult enough to choose the optimal treatment regimens for eradicating H. pylori infection. The most common causes of treatment failure are antibiotic resistance caused by frequent and unregulated usage, as well as a high prevalence of antibiotic side effects. To increase the rate of H. Pylori eradication, a variety of approaches are developed, such as prolonging the treatment period, utilizing new antibiotics, or including natural anti-H. Pylori food products like Cranberry in the treatment [67,68].
The anti-H. pylori characteristics of Cranberry may be accomplished through multiple modes of action, such as bacterial growth inhibition, enzyme inhibition such as urease as well as proline dehydrogenase, anti-adhesion activity, morphological changes, and inhibition of inflammatory cytokines secretion including IL-8 from gastric cells induced by H. pylori [68,69].
According to a study conducted in Gorgan, Iran, the addition of Cranberry to the proton pump inhibitor-based triple therapy for H. pylori had a significant and greater rate of eradication than the usual regimen alone. H. pylori positive patients with peptic ulcer disease were randomly assigned to one of two groups: the first one received a two weeks triple therapy consisting of 30 mg lansoprazole bid, 0.5 g clarithromycin bid, and 1 g amoxicillin bid, while the second group received two weeks 0.5 g Cranberry capsules bid along with the same triple therapy. Bacterial eradication has been assessed using a 13C-urea breath test six weeks after the completion of the treatment. In the first group, H. pylori eradication was achieved in 74% while the percent was 89% in the second group [67].
Dental caries is a multifactorial ailment induced by acid-producing bacteria that are entrenched in the biofilm of dental plaque and ferment dietary carbohydrates like sucrose [70]. Enamel demineralization occurs when the pH of the tooth's surface falls below 5.5, resulting in tooth decay. Due to its aciduric, acidogenic, and adhesion capabilities, Gram-positive bacteria phenotype, primarily the mutant versions of streptococci mainly Streptococcus mutans and Streptococcus sobrinus is thought to be the primary causative organism of dental caries [71,72].
The influence of Cranberry's PACs on the formation, persistence, and development of dental biofilm has been studied. Cranberry PACs' capacity to suppress the activity and synthesis of Glucosyl Transferase (GTF) and Fructosyl Transferase (FTF), which are involved in the formation of exopolysaccharides by S. mutans, has been attributed to their ability to prevent sucrose-dependent biofilm development [73]. Furthermore, Cranberry PACs can prevent bacterial coaggregation, diminish bacterial hydrophobicity, and change cell surface molecules to hinder non-sucrosedependent biofilm formation [74]. In a human trial, it is concluded that the daily usage of Cranberry-containing mouthwash for six weeks can reduce counts of the mutant streptococcal versions in saliva [75].
Another study conducted on children; Gupta et al. revealed that the presence of NDM in the mouthwash can significantly reduce the Streptococcal count in the oral environment [72]. Cranberry PACs have also shown a promise role in the prevention and treatment of periodontal diseases involving gingivitis and periodontitis. Cranberry extract has been demonstrated to diminish periodontopathogen-induced inflammation by lowering inflammatory cytokines, including IL-1, IL-6, IL-8, as well as TNF-α in macrophages [65,74].
Antifungal effect: Fungal infections are becoming more common at an alarming rate, posing a significant challenge to the healthcare providers. This rise is directly linked to the growing number of immune compromised people in addition to the changes in medical practice, such as the use of immunosuppressive medicines and intense chemotherapy. The health problems attributed to pathogenic fungi have also been exacerbated by HIV and other disorders that produce immunosuppression. In addition, many fungal phenotypes are becoming more resistant to traditional antifungal drugs like fluconazole and amphotericin B. These two factors deepen the need for finding potent alternatives, primarily from natural sources [76,77].
Cranberry's capacity to suppress the growth of opportunistic human fungal pathogens that cause oral, respiratory, cutaneous, and systemic infections has recently gotten more attention [78]. Isolated Cranberry proanthocyanidin oligomers can suppress the growth of a variety of human pathogenic fungus species. Candida krusei, Candida glabrata, Cryptococcus neoformans, and Candida lusitaniae all showed vulnerability to micromolar quantities of PAC fractions extracted from Cranberry fruit. Higher antifungal activity was seen as PAC oligomers concentration increased, which could be attributed to an increase in the number of free hydroxyl groups available for interaction with proteins involved in organism development and viability [79].
Furthermore, Rane and his colleagues reported a remarkable in-vitro action of Cranberry PACs against the formation of C. albicans biofilm in artificial urine. Where anti-adherence characteristics, iron chelation, or both are thought to be responsible for the Cranberry PAC action against C. albicans biofilm development [78]. In the context of dental caries, Cranberry extract has reported to suppress cariogenic pathogenicity features of S. mutans and C. albicans dual-species biofilms in an in-vitro model [80].
Metabolic syndrome relieving potential
Metabolic syndrome is a complex disorder characterized by a cluster of cardiovascular risk factors, including central obesity, dyslipidemia, hypertension, impaired glucose metabolism, and associated with a hepatic manifestation represented as Non-Alcoholic Fatty Liver Disease (NAFLD). Consequently, increasing the risk of coronary heart diseases and type 2 diabetes mellitus [81].
Consumption of Cranberry and its-based products may have favorable effects on metabolic syndrome, influencing one or more of its components, a multitude of inflammatory biomarkers, and oxidative stress, as revealed by observational and interventional studies in humans [82].
Through a clinical trial, Lee et al. investigated the effect of Cranberry extract on lipid profiles in Type 2 diabetic patients. The patients who received Cranberry extract supplement, one capsule of 500 mg Cranberry powder after each meal for 12 weeks, exhibited lowering in the atherosclerotic cholesterol profiles. Total cholesterol, Low-Density Lipoprotein (LDL) cholesterol, and the total High-Density Lipoprotein (HDL) cholesterol ratio all decreased considerably in the Cranberry supplemented patients as compared to the placebo patients. Also, Cranberry supplements have a neutral effect on glycemic control in Type 2 diabetic subjects taking oral glucose-lowering agents [83].
The increasing prevalence of metabolic syndrome has been linked to a hepatic manifestation known as Non-Alcoholic Fatty Liver Disease (NAFLD). For instance, a veterinary study was conducted to estimate the hepatoprotective effect of Cranberry using a model, in which, the nonalcoholic fatty liver disease was induced in rats administered either or both of high fat cholesterol diet and Cranberry bulk supplement (50 and 100 mg/ kg/day, three times a week orally for eight weeks). Cranberry supplement dramatically decreased oxidative stress, hepatic steatosis, inflammatory state by lowering the expression of IL-6, TNF-α, and NF-kB. This nutraceutical also enhanced insulin sensitivity and markedly increased the expression of adiponectin. Most importantly, this study revealed that Cranberry may increase Nrf-2 expression in hepatic tissues and considerably reduce collagen formation and expression of fibrotic markers (TGF-β and α-SMA) in a NAFLD model, indicating that Cranberry can significantly reduce liver fibrosis development [84].
Parallel results have been found through a randomized clinical trial conducted by Hormoznejad et al., suggesting that 288 mg of Cranberry extract, which is equivalent to 26 g of dried Cranberry, might improve the management of NAFLD. This is attributable in part to the improvement of insulin resistance and the reduction of serum levels of alanine aminotransferase [85].
In a scope similar to the previous studies, many clinical trials found that eight weeks of Cranberry juice consumption may improve the antioxidant status and reduce the cardiovascular disease risk factors. This can be achieved by enhancing gluco-regulation with a positive impact on the insulin resistance homeostasis model. In addition, Cranberry was ably downregulating inflammatory biomarkers, increasing HDL cholesterol, lowering fasting serum triglycerides, decreasing serum C-reactive protein, and lowering diastolic blood pressure. Accordingly, it has been concluded that Cranberry juice consumption can resolve some cardiovascular risk factors in the disorder named metabolic syndrome [86-89].
On the other hand, Cranberry anthocyanins can modulate the plasma lipoprotein profile by lowering total plasma cholesterol, non-HDL cholesterol and non-HDL cholesterol/HDL-cholesterol ratio. The cholesterol-lowering effect of Cranberry anthocyanins is primarily mediated by the increased excretion of total neutral and acidic faecal sterols [90,91].
According to Xie et al., Cranberry anthocyanins can possess an effective pancreatic lipase inhibiting activity, a well-known target of orlistat. In this study, ultrafiltration was offered as an efficient approach for screening pancreatic lipase inhibitors. Four Cranberry anthocyanins involving cyanidin- 3-O-arabinoside, peonidin-3-O-arabinoside, cyanidin-3-O-galactoside and, peonidin-3-O-galactoside were identified as potent pancreatic lipase inhibitors. The results gathered from molecular dynamics, molecular docking, circular dichroism spectroscopy, and fluorescence spectroscopy illustrated that the aforementioned Cranberry anthocyanins could interact with the active site of pancreatic lipase. Hinting that Cranberry supplement can be exploited to prevent the absorption of lipids and facilitate the treatment and prevention of obesity [92].
Antioxidant potential
The lack of equilibrium, between the incidence of Reactive Species (ROS/ RNS) and the organism's ability to counteract their induced damage via the antioxidative defence systems, was characterized as oxidative stress. This overwork is caused by an increase in the ROS/RNS production or a decrease in the antioxidant protection capacity. As a result, endogenous systems' ability to combat oxidative attacks directed at target biomolecules is diminished [93].
Specific factors that cause oxidative damage in cells may be triggered by oxidative stress, such as the formation of mutagenic compounds, overexpression of oncogenes, inflammation, or promotion of atherogenic activity. Consequently, the risk of cardiovascular diseases, neurodegeneration, carcinogenesis, diabetes, liver diseases, and kidney diseases increased [94,95].
Antioxidants are bioactive molecules that can delay or prevent oxidative damage. They are effective when they prevent oxidative damage by blocking free radical-initiated chain reactions and eliminating them. Aside from the endogenous antioxidant enzymes including catalase, glutathione reductase, superoxide dismutase, and glutathione peroxidase, the antioxidant consumption through the diet or supplements is an additional protective element in maintaining cellular redox homeostasis. To quench the damaging free radicals, the endogenous and exogenous antioxidant defense systems can interact and operate synergistically [96].
Cranberries have been shown to exhibit a beneficial effect on oxidative stress-related disorders. Using HepG2 cells, Martín and coworkers investigated the hepatoprotective effect of Cranberry juice and powdered Cranberry extract in the face of oxidative stress. Pre-treatment of HepG2 cells with the powders for twenty hours resulted in a significant reduction of cellular damage induced by tert-butyl hydroperoxide (t-BOOH). Both powders have considerably reduced the over-activated glutathione peroxidase, glutathione reductase, and Malondialdehyde (MDA). Although the juice powder has a lower antioxidant activity than the extract, it can modulate protein signaling pathways at the molecular level to prevent cell damage. Manifesting that, Cranberry chemo-constituents may preserve hepatocytes from oxidative stress by altering GSH levels, antioxidant enzyme activity, MDA plus ROS production, and cell signaling pathways [97].
Another study that looked at the protective effects of Cranberry extract against iron-induced hepatic toxicity in rats found that the extract possesses potent hepatoprotective properties against iron sulfate-induced liver injury. These benefits could be attributed to Cranberry's membrane protective action via antioxidant and free radical scavenging activities [98]. Furthermore, the results of a study carried out by Kim and colleagues indicated that Cranberry powder may supply adequate antioxidants to help hypercholesterolemic rats exposed to lipopolysaccharide regain their antioxidant capacity [99].
These findings imply that Cranberry and its based products can be used to serve as a springboard for the development of newer, safer, and more effective free radical scavenging agents.
Anti-inflammatory potential
Inflammation is the body's defence mechanism against infections, chemicals, and toxins. If inflammation becomes a chronic condition, it may play a crucial role in the evolution of various chronic noncommunicable diseases, including cardiovascular disease, metabolic syndrome, neurodegenerative diseases, and some malignancies [100-103]. The nuclear factor-κB (NF- κB) plays an important part in the signal transduction pathways that are involved in the inflammatory disorders, in addition to other mediators [104].
A recent study carried out by Yu et al. revealed that PAC-rich Cranberry extract can inhibit the lipopolysaccharide-induced inflammatory responses in the macrophages of mouse bone marrow and RAW264.7. In both cell phenotypes, the extract significantly reduced the production of proinflammatory mediators COX-2, iNos, Il-6, TNF-α, and Mcp-1. The suggested potential mechanisms include the inhibition of NF-B p65 phosphorylation and acetylation levels of histone H4 in both cell types, as well as an increase in HDAC3 protein production [105]. In an earlier study, Denise and colleagues concluded similar results. The Caco-2/15 intestinal cells pre-incubated with Cranberry extract were found to have a reduced pro-inflammatory mediator TNF-α and IL-6 in addition to a lowered NF-κB activation rate [106].
Antitumor potential
Despite ongoing breakthroughs in chemotherapy research, cancer continues to be a crucial challenge for modern medicinal chemistry, necessitating increased global efforts to produce more effective anticancer medicines with fewer side effects to battle this disease [107].
Natural products have been demonstrated promising chemotherapeutic properties in the treatment of a variety of cancers. phytochemicals or their derivatives are already used in the management of different types of cancer. Cranberry and its based products are thought to have anticancer potential due to their high phytochemicals content including the anthocyanins, flavonoids, and phenolic acids [108,109].
Khairnar et al. investigated the anticancer activity of Cranberry extract against KB (Nasopharyngeal carcinoma) and AW13516 (low to moderately differentiated squamous cell carcinoma of the tongue). The authors found that the extract exhibited good anti-proliferative properties against the KB cell line with no remarkable activity against AW13516 cell line [110].
In the case of ovarian carcinoma, highly pure PAC isolated from Cranberry extract has been reported to inhibit angiogenesis and ovarian cancer viability in vitro. Chemotherapy-resistant human ovarian adenocarcinoma (SKOV- 3) cells were treated with PAC that arrested the cell cycle and induced apoptosis. PAC-1 also inhibited VEGF (Vascular Endothelial Growth Factor) function in endothelial cells in addition to the suppression of pro-survival Pi3K/AKT signaling in both ovarian cancer and endothelial cells, which was favorably linked with the prevention of endothelial tube formation.
Using DU145 (human prostate adenocarcinoma) cells, some studies reported that apoptosis could be induced in these cells in response to the cranberry extract treatment, MacLean and colleagues found that Cranberryinduced in vitro apoptosis of DU145 cells can be accomplished through increased caspase-8 and -9 activities [111,112]. Likewise, a researcher and his team found that the daily consumption of 1500 mg powdered Cranberry fruit for a mean of one month lowered serum PSA (prostate-specific antigen) in patients with prostate cancer as Cranberry constituents can regulate the expression of androgen-responsive genes [113].
Discussion and Conclusion
Several centuries ago, Vaccinium macrocarpon received significant attention for its advantageous effects on humans. The dietary supplements of cranberry have been developed in recent decades as an alternate and convenient dietary source of its phytochemicals. Various Cranberry products contain numerous phytoconstituents such as proanthocyanidins, anthocyanins, flavonols, organic acids, and phenolic acids. The antioxidant, antimicrobial, anti-inflammatory, and antitumor activities found in different in vitro, in in vivo, and clinical studies seem to be due to this diverse and rich array of cranberry phytochemicals. Reviewed data in this study strongly imply that cranberry supplements consumption can help manage metabolic syndrome components and protect against cardiovascular diseases, malignancies, microbial infections affecting oral health, urinary tract, and Helicobacter pylori-induced ulcers. Overall, this paper concluded that cranberry extract can be exploited as a viable and promising tool to combat a wide range of human diseases.
References
- Cragg, Gordon M and David J Newman. “Natural Products: a Continuing Source of Novel Drug Leads.” Biochim Biophys Acta 1830 (2013): 3670-3695.
- Licciardi, Paul V and John R Underwood. “Plant-Derived Medicines: A Novel Class of Immunological Adjuvants.” Int Immunopharmacol 11 (2011): 390-398.
- Tran, Ngan, Bao Pham and Ly Le. “Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery.” Biology 9 (2020): 252.
- Jamshidi-Kia, Fatemeh, Zahra Lorigooini and Hossein Amini-Khoei. “Medicinal Plants: Past History and Future Perspective.” J Herbmed Pharmacol 7 (2018): 1-7.
- Mustafa, Yasser, Eman Mohammed and Raghad Khalil. “Synthesis, Characterization, and Anticoagulant Activity of New Functionalized Biscoumarins.” Egypt J Chem 64 (2021): 4461-4468.
- McKay, Diane L and Jeffrey B Blumberg. “Cranberries and Cardiovascular Disease Risk Factors.” Nutr Rev 65 (2007): 490-502.
- Côté, JS. Caillet, G Doyon, JF. Sylvain and M Lacroix. “Bioactive Compounds in Cranberries and Their Biological Properties.” Crit Rev Food Sci Nutr 50 (2010): 666-679.
- Redmond, Elaine. Murphy, J Leonard, K Faulds, and S Abdelfadil, etal. “The Influence of Dietary Supplementation with Cranberry Tablets on the Urinary Risk Factors for Nephrolithiasis.” World J Urol 37 (2019): 561-566.
- Philip, Nebu and Laurence J Walsh. “Cranberry Polyphenols: Natural Weapons Against Dental Caries.” Dent J 7 (2019): 20.
- Krenn, LM Steitz, C Schlicht, H Kurth and F Gaedcke. “Anthocyanin-and Proanthocyanidin-Rich Extracts of Berries in Food Supplements–Analysis with Problems.” Pharmazie 62 (2007): 803-812.
- Wang, Yifei, Peter de B Harrington and Pei Chen. “Analysis of Phenolic Compositions in Cranberry Dietary Supplements Using UHPLC-HRMS.” J Food Compost Anal 86 (2020): 103362.
- Wilson, Ted and Brent A Bauer. “Advising Consumers about Dietary Supplements: Lessons from Cranberry Products.” J Diet Suppl 6 (2009): 377-384.
- Pappas, E and KM Schaich. “Phytochemicals of Cranberries and Cranberry Products: Characterization, Potential Health Effects, and Processing Stability.” Crit Rev Food Sci Nutr 49 (2009): 741-781.
- Côté, J, S Caillet, G Doyon and JF Sylvain, et al. “Analyzing Cranberry Bioactive Compounds.” Crit Rev Food Sci Nutr 50 (2010): 872-888.
- Vvedenskaya, Irina O, Robert T Rosen, Jane E Guido and David J Russell, et al. “Characterization of Flavonols in Cranberry Powder.” J Agric Food Chem 52 (2004): 188-195.
- Oszmianski, Jan, Sabina Lachowicz, Józef Gorzelany and Natalia Matlok. “The Effect of different Maturity Stages on Phytochemical Composition and Antioxidant Capacity of Cranberry Cultivars.” Euro Food Res Technol 244 (2018): 705-719.
- Wu, Xianli and Ronald L Prior. “Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries.” J Agric Food Chem 53 (2005): 2589-2599.
- Blumberg, Jeffrey B, Terri A Camesano, Aedin Cassidy and Penny Kris-Etherton, et al. “Cranberries and their Bioactive Constituents in Human Health.” Adv Nutr 4 (2013): 618-632.
- Viskelis, P, Marina Rubinskiene, Ina Jasutiene and Antanas Šarkinas, et al. “Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry and Their Press Cakes.” J Food Sci 74 (2009): C157-C161.
- Lee, Jungmin. “Anthocyanin Analyses of Vaccinium Fruit Dietary Supplements.” Food Sci Nutr 4 (2016): 742-752.
- Grace, Mary H, Aaron R Massey, Flaubert Mbeunkui and Gad G Yousef, et al. “Comparison of Health-Relevant Flavonoids in Commonly Consumed Cranberry Products.” J Food Sci 77 (2012): H176-H183.
- Mannino, Giuseppe, Vita Di Stefano, Antonino Lauria and Rosa Pitonzo, et al. “Vaccinium Macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed.” Nutrients 12 (2020): 992.
- Wang, Yifei, Ajay P Singh, Heather N Nelson and Amanda J Kaiser, et al. “Urinary Clearance of Cranberry Flavonol Glycosides in Humans.” J Agric Food Chem 64 (2016): 7931-7939.
- Katsargyris, Athanasios, Ekaterini-Christina Tampaki, Constantinos Giaginis and Stamatios Theocharis. “Cranberry as Promising Natural Source of Potential Anticancer Agents: Current Evidence and Future Perspectives.” Anticancer Agents Med Chem 12 (2012): 619-630.
- Gupta, Prachi, Biqin Song, Catherine Neto and Terri A Camesano. “Atomic Force Microscopy-Guided Fractionation Reveals the Influence of Cranberry Phytochemicals on Adhesion of Escherichia Coli.” Food Funct 7 (2016): 2655-2666.
- Neto, Catherine C. “Cranberry and its Phytochemicals: A Review of in Vitro Anticancer Studies.” J Nutr 137 (2007): 186S-193S.
- Lin, Long-Ze, and James M Harnly. “A Screening Method for the Identification of Glycosylated Flavonoids and Other Phenolic Compounds Using a Standard Analytical Approach for all Plant Materials.” J Agric Food Chem 55 (2007): 1084-1096.
- Chen Hao, Zuo Yueagang. “Identification of Flavonol Glycosides in American Cranberry Fruit.” Food Chem 101(2007):1357–64.
- Feliciano, Rodrigo P, Christian G Krueger and Jess D Reed. “Methods to Determine Effects of Cranberry Proanthocyanidins on Extraintestinal Infections: Relevance for Urinary Tract Health.” Mol Nutr Food Res 59 (2015): 1292-1306.
- Seeram, Navindra P, Lynn S Adams, Mary L Hardy and David Heber. “Total Cranberry Extract Versus its Phytochemical Constituents: Antiproliferative and Synergistic Effects Against Human Tumor Cell Lines.” J Agric Food Chem 52 (2004): 2512-2517.
- Smeriglio, Antonella, Davide Barreca, Ersilia Bellocco and Domenico Trombetta. “Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects.” Br J Pharmacol 174 (2017): 1244-1262.
- White, Brittany L, Luke R Howard and Ronald L Prior. “Impact of Different Stages of Juice Processing on the Anthocyanin, Flavonol, and Procyanidin Contents of Cranberries.” J Agric Food Chem 59 (2011): 4692-4698.
- Gu, Liwei, Mark A Kelm, John F Hammerstone and Gary Beecher, et al. “Screening of Foods Containing Proanthocyanidins and their Structural Characterization using LC-MS/MS and Thiolytic Degradation.” J Agric Food Chem 51 (2003): 7513-7521.
- Gardana, Claudio, Antonio Scialpi, Christian Fachechi and Paolo Simonetti. “Identification of Markers for the Authentication of Cranberry Extract and Cranberry-based Food Supplements.” Heliyon 6 (2020): e03863.
- Esquivel-Alvarado, Daniel, Emilia Alfaro-Viquez, Christian G Krueger and Martha M Vestling, et al. “Classification of Proanthocyanidin Profiles using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) Spectra Data Combined with Multivariate Analysis.” Food Chem 336 (2021): 127667.
- Oszmianski, Jan, Joanna Kolniak-Ostek, Sabina Lachowicz and Józef Gorzelany, etal. “Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry.” J Food Sci 82(2017): 2569-2575.
- Chang, Sui Kiat, Cesarettin Alasalvar and Fereidoon Shahidi. “Review of Dried Fruits: Phytochemicals, Antioxidant Efficacies, and Health Benefits.” J Func Food 21 (2016): 113-132.
- Turbitt, John R, Kimberly L Colson, K Brian Killday and Andrew Milstead, et al. “Application of 1H-NMR-based Metabolomics to the Analysis of Cranberry Supplements.” Phytochem Anal 31 (2020): 68-80.
- D'Souza, Doris H. “Phytocompounds for the Control of Human Enteric Viruses.” Curr Opin Virol 4 (2014): 44-49.
- Tripathi, Ashutosh, Sukrat Sinha and Brijesh Kant Dwivedi. “An Attempt to Evaluate Antiviral Activity of Plant Extracts to Combat Infections Caused by Viruses Including SARS COV-2.” 25 (2020):1-23.
- Sekizawa, Haruhito, Kazufumi Ikuta, Katsumi Mizuta and Seiichi Takechi, et al. “Relationship between Polyphenol Content and Anti-Influenza Viral Effects of Berries.” J Sci Food Agric 93 (2013): 2239-2241.
- Weiss, EI, Y Houri-Haddad, E Greenbaum and N Hochman, et al. “Cranberry Juice Constituents Affect Influenza Virus Adhesion and Infectivity.” Antiviral Res 66 (2005): 9-12.
- Oiknine-Djian, Esther, Yael Houri-Haddad, Ervin Itshak Weiss and Itzhak Ofek, et al. “High Molecular Weight Constituents of Cranberry Interfere with Influenza Virus Neuraminidase Activity in Vitro.” Planta Medica 78 (2012): 962-967.
- Neto, CC, KA Penndorf, M Feldman and S Meron-Sudai, et al. “Characterization of Non-Dialyzable Constituents from Cranberry Juice that Inhibit Adhesion, Co-Aggregation and Biofilm Formation by Oral Bacteria.” Food Funct 8 (2017): 1955-1965.
- Luganini, Anna, Maria E Terlizzi, Gianluca Catucci and Gianfranco Gilardi, et al. “The cranberry extract Oximacro Exerts in Vitro Virucidal Activity Against Influenza Virus by Interfering with Hemagglutinin.” Front Microbiol 9 (2018): 1826.
- Terlizzi, Maria Elena, Andrea Occhipinti, Anna Luganini and Massimo E Maffei, et al. “Inhibition of Herpes Simplex Type 1 and Type 2 Infections by Oximacro, a Cranberry Extract with a High Content of A-Type Proanthocyanidins (PACs-A).” Antiviral Res 132 (2016): 154-164.
- Lipson, SM, L Sethi, P Cohen and RE Gordon, et al. “Antiviral Effects on Bacteriophages and Rotavirus by Cranberry Juice.” Phytomedicine 14 (2007): 23-30.
- Su, Xiaowei, Amy B Howell and Doris H D'Souza. “The Effect of Cranberry Juice and Cranberry Proanthocyanidins on the Infectivity of Human Enteric Viral Surrogates.” Food Microbiol 27 (2010): 535-540.
- Li, Dan, Leen Baert, Ming Xia and Weiming Zhong et al. “Effects of a Variety of Food Extracts and Juices on the Specific Binding Ability of Norovirus GII. 4 P particles.” J Food Prot 75 (2012): 1350-1354.
- Su, Xiaowei, Amy B Howell and Doris HD’Souza. “Antiviral Effects of Cranberry Juice and Cranberry Proanthocyanidins on Foodborne Viral Surrogates–a Time Dependence Study in Vitro.” Food Microbiol 27 (2010): 985-991.
- Ryu, Seungbo, Hyun Ju You, Ye Won Kim and Ariel Lee, et al. “Inactivation of Norovirus and Surrogates by Natural Phytochemicals and Bioactive Substances.” Mol Nutr Food Res 59 (2015): 65-74.
- Liu D, Deng J, Joshi S and Liu P, et al. “Monomeric Catechin and Dimeric Procyanidin B2 Against Human Norovirus Surrogates and their Physicochemical Interactions.” Food Microbiol 76 (2018):
- Lipson, Steven M, Fatma S Ozen, Laina Karthikeyan and Ronald E Gordon. “Effect of PH on Anti-Rotavirus Activity by Comestible Juices and Proanthocyanidins in a Cell-Free Assay System.” Food Environ Virol 4 (2012): 168-178.
- Lipson, SM, G Karalis, L Karthikeyan and FS Ozen, et al. “Mechanism of Anti-Rotavirus Synergistic Activity by Epigallocatechin Gallate and a Proanthocyanidin-Containing Nutraceutical.” Food Environ Virol 9 (2017): 434-443.
- Lipson, SM, G Karalis, L Karthikeyan and FS Ozen, et al. “Mechanism of Anti-Rotavirus Synergistic Activity by Epigallocatechin Gallate and a Proanthocyanidin-Containing Nutraceutical.” Food Environ Virol 9 (2017): 434-443.
- Jensen, Heidi D, Carsten Struve, Søren B Christensen and Karen A Krogfelt. “Cranberry Juice and Combinations of its Organic Acids are Effective Against Experimental Urinary Tract Infection.” Front Microbiol 8 (2017): 542.
- Mohammed, Eman Tareq and Yasser Fakri Mustafa. “Coumarins from Red Delicious Apple Seeds: Extraction, Phytochemical Analysis, and Evaluation as Antimicrobial Agents.” Sys Rev Pharmacy 11 (2020): 64-70.
- Howell, Amy B, Henry Botto, Christophe Combescure and Anne-Béatrice Blanc-Potard et al. “Dosage Effect on Uropathogenic Escherichia Coli Anti-Adhesion Activity in Urine Following Consumption of Cranberry Powder Standardized for Proanthocyanidin Content: A Multicentric Randomized Double Blind Study.” BMC Infect Dis 10 (2010): 1-11.
- Ledda, A, G Belcaro, M Dugall and A Riva, et al. “Highly Standardized Cranberry Extract Supplementation (Anthocran®) as Prophylaxis in Young Healthy Subjects with Recurrent Urinary Tract Infections.” Eur Rev Med Pharmacol Sci 21 (2017): 389-393.
- Juthani-Mehta, Manisha, Peter H Van Ness, Luann Bianco and Andrea Rink, et al. “Effect of Cranberry Capsules on Bacteriuria Plus Pyuria Among Older Women in Nursing Homes: A Randomized Clinical Trial.” Jama 316 (2016): 1879-1887.
- Seely D, Dugoua JJ, Perri D and Mills E Koren G. “Safety and Efficacy of Panax Ginseng During Pregnancy and Lactation.” Can J Clin Pharmacol 15 (2008): 80–6.
- Ledda, A, G Belcaro, M Dugall and B Feragalli, et al. “Supplementation with High Titer Cranberry Extract (Anthocran®) for the Prevention of Recurrent Urinary Tract Infections in Elderly Men Suffering from Moderate Prostatic Hyperplasia: A Pilot Study.” Eur Rev Med Pharmacol Sci 20 (2016): 5205-5209.
- Mutlu, Hatice and Zelal Ekinci. “Urinary Tract Infection Prophylaxis in Children with Neurogenic Bladder with Cranberry Capsules: Randomized Controlled Trial.” ISRN Pediatr 12 (2012):20.
- Srinidhi, Kaviya A. “Cranberry and its Antibacterial Activity-A Review.” J Pharmaceutical Sci Res 6 (2014): 41.
- Scharf, Birte, Jandirk Sendker, Ulrich Dobrindt and Andreas Hensel. “Influence of Cranberry Extract on Tamm-Horsfall Protein in Human Urine and its Antiadhesive Activity Against Uropathogenic Escherichia Coli ” Planta Medica 85 (2019): 126-138.
- Seyyedmajidi, Mohammadreza, Anahita Ahmadi, Shahin Hajiebrahimi and Seyedali Seyedmajidi, et al. “Addition of Cranberry to Proton Pump Inhibitor-Based Triple Therapy for Helicobacter Pylori Eradication.” J Res Pharm Pract 5(2016): 248.
- Matsushima, Masashi, Takayoshi Suzuki, Aya Masui and Tetsuya Mine et al. “Cranberry Extract Suppresses Interleukin-8 Secretion from Stomach Cells Stimulated by Helicobacter Pylori in Every Clinically Separated Strain but Inhibits Growth in Part of the Strains.” J Function Foods 5 (2013): 729-735.
- Chidambaram, Sathish Kumar, Daoud Ali, Saud Alarifi and Surendrakumar Radhakrishnan, et al. “in silico Molecular Docking: Evaluation of Coumarin Based Derivatives Against SARS-CoV-2.” J Infect Public Health 13 (2020): 1671-1677.
- Sanz, Mariano, David Beighton, Michael A Curtis and Jaime A Cury, et al. “Role of Microbial Biofilms in the Maintenance of Oral Health and in the Development of Dental Caries and Periodontal Diseases Consensus Report of Group 1 of the Joint EFP/ORCA Workshop on the Boundaries Between Caries and Periodontal Disease.” J Clin Periodontol 44 (2017): S5-S11.
- Quock, Ryan L “Dental Caries: A Current Understanding and Implications.” J Nature Sci 1(2015): 27.
- Gupta, Akanksha, Kalpana Bansal and Mohita Marwaha. “Effect of High-Molecular-Weight Component of Cranberry on Plaque and Salivary Streptococcus Mutans Counts in Children: An in in vivo Study.” J Indian Soc Pedod Prev Dent 33 (2015): 128.
- Koo, H, S Duarte, RM Murata and K Scott-Anne, et al. “Influence of Cranberry Proanthocyanidins on Formation of Biofilms by Streptococcus Mutans on Saliva-Coated Apatitic Surface and on Dental Caries Development in in vivo.” Caries Res 44 (2010): 116-126.
- Fu, Zhuxuan, DeAnn Liska, David Talan and Mei Chung. “Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis.” J Nutr 147 (2017): 2282-2288.
- Howell, Amy B. “Potential of Cranberry for Suppressing Helicobacter Pylori, a Risk Factor for Gastric Cancer.” J Berry Res 10 (2020): 11-20.
- Garber, Gary. “An Overview of Fungal Infections.” Drugs 61 (2001): 1-12.
- Mustafa, Yasser Fakri, Raghad Riyadh Khalil and Eman Tareq Mohammed. “Antimicrobial Activity of Aqueous Extracts Acquired From the Seeds of Two Apples’ Cultivars.” Sys Rev Pharmacy 11 (2020): 382-387.
- Feghali, Karine, Mark Feldman, Vu Dang La and Juliana Santos et al. “Cranberry Proanthocyanidins: Natural Weapons Against Periodontal Diseases.” J Agric Food Chem 60 (2012): 5728-5735.
- Weiss, Ervin I, Avital Kozlovsky, Doron Steinberg and Ron Lev-Dor, et al. “A High Molecular Mass Cranberry Constituent Reduces Mutans Streptococci Level in Saliva and Inhibits in Vitro Adhesion to Hydroxyapatite.” FEMS Microbiol Lett 232 (2004): 89-92.
- Philip, Nebu, Shaneen J Leishman, Hmhn Bandara and Laurence J Walsh. “Polyphenol-Rich Cranberry Extracts Modulate Virulence of Streptococcus Mutans-Candida Albicans Biofilms Implicated in the Pathogenesis of Early Childhood Caries.” Pediatr Dent 41 (2019): 56-62.
- Rane, Hallie S, Stella M Bernardo, Amy B. Howell and Samuel A Lee. “Cranberry-Derived Proanthocyanidins Prevent Formation of Candida Albicans Biofilms in Artificial Urine Through Biofilm-and Adherence-Specific Mechanisms.” J Antimicrob Chemother 69 (2014): 428-436.
- Patel, Kunal D, Frank J Scarano, Miwako Kondo and Robert AR Hurta, et al. “Proanthocyanidin-Rich Extracts from Cranberry Fruit (Vaccinium macrocarpon Ait.) Selectively Inhibit the Growth of Human Pathogenic Fungi Candida spp. and Cryptococcus Neoformans.” J Agric Food Chem 59 (2011): 12864-12873.
- Kowalska, Katarzyna and Anna Olejnik. “Beneficial Effects of Cranberry in the Prevention of Obesity and Related Complications: Metabolic Syndrome and Diabetes–A Review.” J Function Foods 20 (2016): 171-181.
- Faheem, Safaa A, Noha M Saeed, Reem N. El-Naga and Iriny M Ayoub, et al. “Hepatoprotective Effect of Cranberry Nutraceutical Extract in Non-Alcoholic Fatty Liver Model in Rats: Impact on Insulin Resistance and Nrf-2 Expression.” Front Pharmacol 11 (2020): 218.
- Thimóteo, Nataly Simões Bandiera, Bruna Miglioranza Scavuzzi and Isaias Dichi. “The Impact of Cranberry (Vaccinium macrocarpon) and Cranberry Products on Each Component of the Metabolic Syndrome: A Review.” Nutrire 42 (2017): 1-12.
- Lee, IT, YC Chan, CW Lin and WJ. Lee, et al. “Effect of Cranberry Extracts on Lipid Profiles in Subjects with Type 2 Diabetes.” Diabetic Med 25 (2008): 1473-1477.
- Basu, Arpita, Nancy M Betts, Jennifer Ortiz and Brandi Simmons, et al. “Low-Energy Cranberry Juice Decreases Lipid Oxidation and Increases Plasma Antioxidant Capacity in Women with Metabolic Syndrome.” Nutr Res 31 (2011): 190-196.
- Hormoznejad, Razie, Majid Mohammad Shahi, Fakher Rahim and Bijan Helli, et al. “Combined Cranberry Supplementation and Weight Loss Diet in Non-Alcoholic Fatty Liver Disease: A Double-Blind Placebo-Controlled Randomized Clinical Trial.” Int J Food Sci Nutr 71 (2020): 991-1000.
- Chew, Boon, Bridget Mathison, Lindsey Kimble and Diane McKay, et al. “Chronic Consumption of a Low Calorie, High Polyphenol Cranberry Beverage Attenuates Inflammation and Improves Glucoregulation and HDL Cholesterol in Healthy Overweight Humans: A Randomized Controlled Trial.” Eur J Nutr 58 (2019): 1223-1235.
- Hamer, Mark and Gita Mishra. “Role of Functional Foods in Primary Prevention: Cranberry Extracts and Cholesterol Lowering.” Clin Lipidology 4 (2009): 141-143.
- Novotny, Janet A, David J Baer, Christina Khoo and Sarah K Gebauer, et al. “Cranberry Juice Consumption Lowers Markers of Cardiometabolic Risk, Including Blood Pressure and Circulating C-Reactive Protein, Triglyceride, and Glucose Concentrations in Adults.” J Nutr 145 (2015): 1185-1193.
- Lozovoy, Marcell Alysson Batisti, Sayonara Rangel Oliveira, Danielle Venturini and Helena Kaminami Morimoto, et al. “Reduced-Energy Cranberry Juice Increases Folic Acid and Adiponectin and Reduces Homocysteine and Oxidative Stress in Patients with the Metabolic Syndrome.” Br J Nutr 110 (2013): 1885-1894.
- Khalil, Raghad Riyadh and Yasser Fakri Mustafa. “Phytochemical, Antioxidant and Antitumor Studies of Coumarins Extracted from Granny Smith Apple Seeds by different Methods.” Syst Rev Pharm 11 (2020): 57-63.
- Wang, Lijun, Hanyue Zhu, Yimin Zhao and Rui Jiao, et al. “Cranberry Anthocyanin as an Herbal Medicine Lowers Plasma Cholesterol by Increasing Excretion of Fecal Sterols.” Phytomedicine 38 (2018): 98-106.
- Mustafa, Yasser Fakri, Eman Tareq Mohammed and Raghad Riyadh Khalil. “Antioxidant and Antitumor Activities of Methanolic Extracts Obtained from Red Delicious and Granny Smith Apples' Seeds.” Sys Rev Pharmacy 11 (2020): 570-576.
- Pisoschi, Aurelia Magdalena and Aneta Pop. “The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review.” Eur J Med Chem 97 (2015): 55-74.
- Martin, Maria Angeles, Sonia Ramos, Raquel Mateos and Jannie PJ Marais, et al. “Chemical Characterization and Chemo-Protective Activity of Cranberry Phenolic Powders in a Model Cell Culture. Response of the Antioxidant Defenses and Regulation of Signaling Pathways.” Food Res Int 71 (2015): 68-82.
- Hussien, A-MA, MA Hussein, AD-A El Mageed and AM Abdel-Baky. “Cranberry Extract as a Functional Food in Treatment of Oxidative Stress in Iron-Induced Hepatic Toxicity in Rats.” J Drug Metab Toxicol 6 (2015): 2.
- Xie, Lianghua, Jiahong Xie, Yang Xu and Wei Chen. “Discovery of Anthocyanins from Cranberry Extract as Pancreatic Lipase Inhibitors Using a Combined Approach of Ultrafiltration, Molecular Simulation and Spectroscopy.” Food Funct 11 (2020): 8527-8536.
- Cichoz-Lach, Halina and Agata Michalak. “Oxidative Stress as a Crucial Factor in Liver Diseases.” World J Gastroenterol 20 (2014): 8082.
- Kim, Mi Joung, Jung Hee Kim and Ho-Kyung Kwak. “Antioxidant Effects of Cranberry Powder in Lipopolysaccharide Treated Hypercholesterolemic Rats.” Prev Nutr Food Sci 19 (2014): 75.
- Wyss-Coray, Tony and Joseph Rogers. “Inflammation in Alzheimer Disease—a Brief Review of the Basic Science and Clinical Literature.” Cold Spring Harb Perspect Med 2 (2012): a006346.
- Monteiro R, Azevedo I. "Chronic Inflammation in Obesity and the Metabolic Syndrome.” Mediators Inflamm (2010):1–10.
- Oglah, Mahmood Khudhayer, Yasser Fakri Mustafa, Moath Kahtan Bashir and Mahmood Hashim Jasim et al. “Curcumin and its Derivatives: A Review of their Biological Activities.” Syst Rev Pharm 11 (2020): 472.
- Yu, Seok-Yeong, Jungbae Oh, Justin S Kim and Young-In Kwon, et al. “Anti-Inflammatory Effects of Proanthocyanidin-Rich Cranberry Extract through the Suppression of NF-kB Pathway and Histone Acetylase in RAW 264.7 and Mouse Bone Marrow-derived Macrophages.” J Food Nutr Res 9 (2021): 79-86.
- Amor, Sandra, Fabiola Puentes, David Baker and Paul Van Der Valk. “Inflammation in Neurodegenerative Diseases.” Immunology 129 (2010): 154-169.
- Mustafa, Yasser, Raghad Riyadh Khalil, and Eman Tareq Mohammed. “Synthesis and Antitumor Potential of New 7-Halocoumarin-4-Acetic Acid Derivatives.” Egypt J Chem 64 (2021): 3711-3716.
- Libby, Peter. “Inflammation and Cardiovascular Disease Mechanisms.” Am J Clin Nutr 83 (2006): 456S-460S.
- Oglah, Mahmood Khudhayer, Moath Kahtan Bashir, Yasser Fakri Mustafa and Eman Tareq Mohammed, et al. “Synthesis and Biological Activities of 3, 5-Disubstituted-4-Hydroxycinnamic Acids Linked to a Functionalized Coumarin.” System Rev Pharmacy 11(2020): 717-725.
- Khairnar, Mahesh R, Umesh Wadgave, Harish Jadhav and Rahul Naik. “Anticancer Activity of Chlorhexidine and Cranberry Extract: An in-Vitro Study.” J Exp Ther Oncol 12 (2018): 201-205.
- Denis, Marie-Claude, Yves Desjardins, Alexandra Furtos and Valérie Marcil, et al. “Prevention of Oxidative Stress, Inflammation and Mitochondrial Dysfunction in the Intestine by Different Cranberry Phenolic Fractions.” Clinical Sci 128 (2015): 197-212.
- Déziel, Bob, James MacPhee, Kunal Patel and Adriana Catalli, et al. “American Cranberry (Vaccinium Macrocarpon) Extract Affects Human Prostate Cancer Cell Growth Via Cell Cycle Arrest by Modulating Expression of Cell Cycle Regulators.” Food Funct 3 (2012): 556-564.
- Student, Vladimir, Ales Vidlar, Jan Bouchal and Jana Vrbkova, et al. “Cranberry Intervention in Patients with Prostate Cancer Prior to Radical Prostatectomy. Clinical, Pathological and Laboratory Findings.” Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 160 (2016): 559-565.
Citation: Khalil Raghad Riyadh, Eman Tareq Mohammed and Yasser Fakri Mustafa. "Various Promising Biological Effects of Cranberry Extract: A Review" Clin Schizophr Relat Psychoses 15S(2021). Doi: 10.3371/ CSRP.KRET.113021.
Copyright: © 2021 Khalil RR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.