Case Study - Clinical Schizophrenia & Related Psychoses ( 2022) Volume 0, Issue 0
Unique Presentation of Neurosarcoidosis: Case Report and Systematic Review
Humberto Foyaca-Sibat, Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 21-Jan-2022, Manuscript No. CSRP-21-52000; Editor assigned: 24-Jan-2022, Pre QC No. CSRP-21-52000 (PQ); Reviewed: 14-Feb-2022, QC No. CSRP-21-52000; Revised: 16-Feb-2022, Manuscript No. CSRP-21-52000 (R); Published: 23-Feb-2022, DOI: 10.3371/CSRP.SF.022322
Abstract
Background: Sarcoidosis (Sa) is an uncommon and poorly known inflammatory multisystem disease of uncertain aetiology, histopathological characterized by noncaseating giant-cell granulomas affecting the lungs, the heart, peripheral nervous system, and the eyes mainly. Neurologic involvement of Sa (NS) can involve the central nervous system or peripheral nervous system or both simultaneously, causing remarkable morbidity and mortality if NS remains untreated. Up to date, there is no worldwide agreed diagnostic program for patients with Sa.
Case presentation: A 37-year-old female patient complained of chronic headache, upper back pain, and progressive decreasing visual acuity since 2012. In May 2021, she was admitted to the ophthalmology ward because her visual acuity deteriorated, and she lost vision on the right eye three weeks after admission. She also complained of generalized intermittent headache and dizziness, not associated with other symptoms or signs, without aggravating or realizing factors but responding to routinary pain killers. HIV non-reactive with PCR for COVID-19 negative. The diagnosis of ON in the right eyes and optic peri-neuritis in the left eyes was made. Neuro-ophthalmologic examination confirmed ON and OP. Investigations confirmed EST, ON, OP and IDEM granulomas.
Results: Only one male patient presented NS/ON/OP was identified through this searching research. Therefore, our case is the first one affected by ON/OP/EST/IDEM/NS to be reported to the international medical community.
Discussion: After a powerful searching of the literature, we did not find a case of NS combining ON/OP with empty sellar turcica syndrome and spinal tumour. Therefore, this patient is the first reported to the medical literature with such presentation from our knowledge. We discuss on the Histopathology and differential diagnosis of NS, Disorder of the sella turcica, ocular sarcoidosis, treatment, and mortality rates. Finally, we delivered one hypothesis on pathogenesis of Sa granuloma formation.
Conclusion: We delivered a hypothesis on the pathogenesis of the NS based on the role played by TNF-α, IFN-β/γ, and IL-2, dysbiosis, and other elements the mechanism of Sa granuloma formation. Finally, we concluded that this literature search might serve other investigators to comprehend better the NS, the importance of making an early diagnosis with therapy and performing a regular assessment looking for other frequently associated disorders and their management. Notwithstanding, large, and well-designed cross-section studies and RCT will be necessary to provide the best information about all this matter.
Keywords
Neurosarcoidosis; Empty sellar turcica; Sarcoid intradural extramedullary tumour; Opticneuritis; Optic perineuritis
Abbrevations
COVID-19: Coronavirus disease of 2019; CRP: C reactive protein; TLC: Total Leucocyte Count; CT: Computed Tomography; Sao2: Oxygen Saturation
Introduction
Sarcoidosis (Sa) is an uncommon and poorly known inflammatory multisystem disease of uncertain aetiology, which is histopathological characterized by noncaseating giant-cell granulomas affecting the lungs, the heart, peripheral nervous system, the eyes, liver, joints, spleen, tongue, gastrointestinal tract, paranasal sinuses, bilateral parotitis, nasosinusal or laryngeal signs, hypercalcemia, renal dysfunction, aseptic meningitis, cerebral parenchymal disease, breast, and skeletal muscles. However, in 90% of cases, Sa focuses mainly on the intrathoracic lymph nodes and lungs. Nevertheless, virtually any organic system can be affected, leading to neurological signs, blindness, or death. For most cases, the medication of choice is corticosteroids, immunosuppressive and biologic agents offering fast and durable remissions [1]. Some clinical features are accepted as highly specific of the disease (e.g., lupus pernio, Löfgren's syndrome and Heerfordt's syndrome); in these cases, histological confirmation is not required. The first description of Sa was made by Sir Jonathan Hutchinson in 1877, while Heerfordt has described the uveoparotid fever in 1917, which is considered the first description of neurosarcoidosis (NS) [2].
Neurologic involvement of Sa (NS) can involve the central nervous system (CNS) or peripheral nervous system (PNS) or both at the same time causing remarkable morbidity. More specifically, between 5% to 10% of total cases of NS show significant involvement of the nervous system (NES), and from them, 70%-90% of cases have extra neural involvement from the beginning of the disease or even during its progression. It has been documented that more than 15% of patients have a fatal prognosis [3]. The most common places of the NS affected by NS are the hypothalamus- pituitary axis, brain parenchyma, cranial nerves, meninges, spinal cord, and peripheral nerves. Usually, these manifestations appear between the first two years from the beginning of Sa. The seventh cranial nerve is the most affected (15%-39%), followed by optic nerves (4%-25%), the fifth cranial nerves (8%-19%) and the vestibulocochlear nerve. The optic nerve damage can be secondary to intracranial hypertension (papilloedema), direct injury of the nerves (optic neuritis (ON)/optic peri neuritis (OP) or even the optic chiasma leading to unilateral or bilateral permanent blindness, visual disturbances and hemianopias and communicating Hydrocephalus. On the other hand, lesions of the VIII cranial nerve cause subjective vertigo and hearing loss, while secondary trigeminal neuralgia can be a consequence of trigeminal damage [3,4].
Other authors reported cases of NS presenting hormonal dysfunction caused by hypoadrenalism, syndrome of inappropriate antidiuretic hormone, and hypothyroidism secondary to pituitary/hypothalamic axis damage [5,6]. When NS affects the CNS, epilepsy, chronic headaches, cognitive decline, strokes, dilatation of the ventricular system and abnormal gaits can be observed [3]. In 12%-40% of cases of NS, meningeal signs are present and clinical manifestations of space-occupying lesions (20%-45%) can be documented [3,4]. Involvement of the peripheral nerves has been reported in 4%-17% of affected cases by NS, and the common pathological finding was granulomatous peri neuritis or vasculitis [3].
In the meantime, non-investigators have proved why the NS can affect the spinal cord (SC) at any level of the intramedullary or intra/extradural extramedullary space and not convince explanation for that has been delivered up to date, despite other authors have been reported neurological signs from the SC involvement such as motor or sensory deficits, sexual dysfunction, and bladder/bowel disorders [7,8]. In addition, fatigue and cognitive decline are common in the case of sarcoidosis (with or without NS) [2].
Commonly, NS shows a benign prognosis with a considerable improvement or stability at long-term follow-up (around 2 to 3 years), influenced by demographic features that determine its clinical course, topographic of the lesions, and outcome [9]. In 1993, the mortality rate was reported very low worldwide, and in Japan, 77% of patients with sarcoidosis died due to cardiac involvement, while in Europe and North America, more than 80% of reported death was due to respiratory complications [10]. Now, the correlation is unknown.
One of the most significant case series reports was made by Dorman who reported 1706 cases, including 82 cases (4.8%) of NS; among this group, 89% of cases were African American peoples. In their study, 21.7% of cases presented spinal cord disorders, followed by 16.0% of ON/CH involvement, 13.3% of seizure disorders. NS's most common clinical presentation is facial nerve palsy in most cases. By curiosity, this cohort differs from others reported in the medical literature due to the low prevalence of seventh cranial nerve involvement. Chest x-ray showed signs of sarcoidosis in 43.3% of their series and chest CT in 78.6% of cases [11]. The prevalence of Sa is 60 per 100,000 worldwide, which age of onset remains around 20 to 40 years [2]. The clinical manifestations of Sa are still a great challenger because of its capacity to mimic many other conditions with different courses like acute, chronic, monophasic, relapsing, remittent, progressing and so forth. Apart from the increased incidence of Sa in African Americans, Caribbean Americans, and Scandinavians. Nevertheless, only the first group presented a higher involvement of the extra thoracic regions [12-16]. The list of the pathological process to be included in the broad differential diagnosis includes infectious granulomatous diseases, demyelinating diseases, fungal infections, malignancies, vasculitis or neurosyphilis [11].
Up to date, there is no worldwide agreed diagnostic program for patients with Sa. However, there is a general programmed approach to exclude infectious vasculitis and CT/MRI of the neuroaxis similarities through a serological and microbiologic assessment followed by chest x-rays/CT chest, imagenology of the NS, a biopsy of the lung/lymph nodes in the mediastinum, PET-CT, and Gallium scintigraphy. In all patients presenting spinal cord lesions, MRI studies of the SC confirmed the diagnosis of NS. In the study done by Dorman no pathological findings at the lumbosacral area were found [11]. However, Soohn found a substantial number of cases (37%) [17]. Investigations on the CSF showed average results in 23% of cases; other findings were nonspecific, including oligoclonal band when present. In addition, CSF hypoglycorrhachia was absent in some reports, while this result is at odds in other reports, giving valuable support in cases with an associated meningeal syndrome, Hydrocephalus, and SC compression. However, several patients with pachymeningitis had a normal CSF, which means that abnormalities found in the CSF may not reflect the level of damage of the CNS in patients with NS [18].
Novel expectations have been arising with the introduction of TNFα-blockers (infliximab) [16, 19] and adalimumab [16,20,21]. In a case with a refractory response, Obviously, despite the rarity of this condition, an extensive and well-designed prospective controlled trials investigation will be necessary to support or reject this expectation.
On the other hand, other authors, after reporting a case of a 44-yearold African American man presenting an intramedullary expansile patchy SC lesion in the cervicothoracic segments which diagnosis was confirmed by paratracheal lymph-node biopsy they recommended high suspicion of NS when African American patients in the age group of 20-50 years consulting for SC symptomatology because early treatment prevents further progression and fatal outcome [22].
Regarding the same topic about CS involvement by NS, Longo reported a case of 46-year-old female complaining of lower back pain, abnormal gait, uncontrolled bladder, and sensory loss with normal muscle strength. Her spine MRI confirmed multiple epidural lesions leading to SC compression at the T5 level. She received dexamethasone followed by prednisone showing a complete neurological recovery and was gainfully employed without a surgical approach which confirms that in some instances of epidural, NS medical treatment alone can be enough [23]. On the other hand, it has been confirmed that SC lesions by Sa occur in approximately 10% of overall cases of NS, affecting mainly the intramedullary region like transverse myelitis [24-28]. Extramedullary/intradural NS is exceedingly rare, and epidural NS is even less frequent [29-33].
In a review of the medical literature made by Longo, there found only four reported cases with epidural NS presenting an SC disorder without systemic manifestations; all of them required a surgical approach the forestall the disease [32-35]. One of these cases was a 44-year-old lady who presented with sensory disturbance in the power limbs, and her MRI showed a T2 hyperintense lesion at the thoracolumbar junction. The biopsy confirmed a non-necrotizing granulomatous inflammation. She was treated with corticosteroids and underwent a T11-L1 laminectomy with completed recovery [35]. Another report refers to a 52-year-old lady presenting spastic paraparesis and sensory loss at the T4 level. Imaging showed a contrast- enhancing tumour at the T2-T6 level occupying the whole spinal canal. She also got a laminectomy with corticosteroid therapy and recovery fully [34]. The third report refers to a 37-year-old male with cauda equina syndrome due to a sizeable epidural sarcoid granuloma from L1-S1 who recovered completely after steroid therapy and surgical intervention [33]. The last report was a 9-year-old girl who presented SC manifestations secondary to recurrent epidural disorder caused by epidural NS who underwent multiple surgical interventions and several courses of corticosteroids therapy with final improvement [32]. Finally, the Longo report differs from the previous ones because their patient improved without surgical approach despite signs of SC compression, proving that patients affected by SC compression secondary to epidural NS can achieve full recovery with medical treatment only [23].
As a general acceptance, the infiltration of the SC parenchyma, extradural space, leptomeninges or extraspinal structures is the most ordinary way to affect the SC in 19%-26% of patients with NS [27,36,37]. Commonly findings on imaging investigations include linear leptomeningeal contrast enhancement, nodular lesions and associated intraparenchymal T2 hyperintensity [38,39]. One of the previously published series showed that more than 74% of patients presenting NS were involved in ≥ 3 vertebral segments of the SC represented by extensive longitudinal myelitis (LETM) [38]. To be sure that LETM is caused by NS and not from another aetiology, the MRI finding must include a posterior SC subpial pattern of gadolinium enhancement from ≥ 2 segments of the cord and that enhancement should persist for more than two consecutive months despite the treatment or subpial enhancement of the dorsal column and central canal resembling a trident on axial MRI which is also a sign of higher suspicion for NS in cases presenting subacute myelitis [40,41].
In 2020, Murphy identified four groups of NS of the SC on MRI, being the commonest one the LETM (45%), followed by meningoradiculitis/ meningitis (23%), short tumefactive myelitis (23%), and anterior myelitis next to disc degeneration (10%). In addition, the same author established that MRI gadolinium enhancement could be present, and the most affected regions of the SC are cervicothoracic, although conus medullaris and cauda equina may be affected as well [42].
For the list of types of NS lesions on the SC, other authors include maslike dural and leptomeningeal lesions, perivascular and leptomeningeal lesions, short segment myelitis, and lumbosacral radiculitis (Elsberg syndrome) [37, 39-41]. The 15 years retrospective study made by Soni reported that 72% of cases with NS presented SC involvement (13/18), including leptomeningeal enhancement (61%), intramedullary enhancing lesions (38%), pachymeningeal (23%), and bony involvement (15%) [43]. The cervical region was most frequently involved, followed by the thoracic area. More often, affecting large regions (4.2 spinal segments) with predominant involvement of the dorsal cord-like previous publications. Four cases had isolated CNS involvement in this series, including one with isolated SC involvement. Authors concluded that despite Sa of SC being previously considered a rare disorder, it is now increasingly recognized with the widespread use of MRI studies. The predominance of dorsal column involvement is characteristic but not necessarily pathognomonic [43].
Intradural extramedullary (IDEM) NS is considered a rare disorder by Ishiwata, collaborators [43] and other authors [44-46]. They reported a 32-year-old man complaining of clumsy hands, paresthesias in the trunk and paraparesis. The MRI scan revealed a cervical tumour at the IDEM, and a meningioma was suspected.
The patient underwent surgical treatment for total resection of the mass lesion, and the histopathological study confirmed a sarcoid lesion. Fortunately, the patient reached a complete recovery [43].
Other authors confirmed the rarity of involvement of the SC by NS [47- 54]. However, recently other authors delivered more information on NS of the SC and confirmed its frequency is less than 1 % of all reported cases up to date [55-60].
Recently, Yan Lin reported a 51-year-old male presenting NC with ON and OP simultaneously. They could rule out other inflammatory conditions causing ON/OP like spatially distinct optic neuritis and myelin oligodendrocyte antibody-associated disease. They finally documented this patient as the first case report of OPNS in the medical literature up to date [61].
Apart to report a highly uncommon medical case, the primary aid of this article is answering two review research questions. 1. How often is the combination of optic neuritis/optic peri neuritis, empty sellar turcica, intradural extramedullary tumour of the spine in patients presenting neurosarcoidosis? What is the pathogenesis of NS?.
Literature search strategy
We included case reports, case series, observational cohort studies, systematic review and meta-analysis, cross-sectional studies, and clinical trials on the association of ON, OP, EST, IDEMNS in patients with NS. During the initial search, we looked for all articles published between December 01, 2014, and December 01, 2021. We search the following databases: Medline, Scopus online databases, Google Scholar, Science Direct, Scielo, and medRxiv. All Studies were retrieved by utilizing medical subject headings (MeSH).
All items about "neurosarcoidosis", OR "spinal cord NS" OR "optic neuritis NS" OR "empty sella turcica syndrome NS" OR "optic peri neuritis NS", OR ITEMS. Alternatively, NS/ON/OP/EST/IDEM*, where * is the PubMed Central wild card for every possible word beginning or ending. We did not include other clinical presentations beyond the current work scope.
Study and cohort selection
We select prospectively and retrospective cohort studies, case reports, case series, case-control studies, controlled clinical trials, review, and meta-analysis reporting data on NC/ONNC/OPNC/ESTNC/IDEMNC.
Study selection
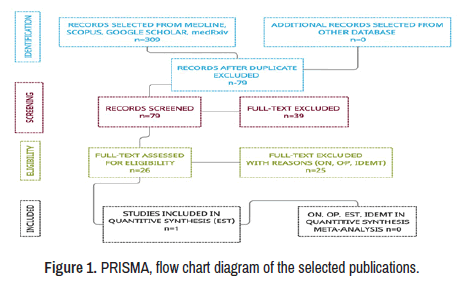
This study aims at the prevalence of NC/ONNC/OPNC/ESTNC/ IDEMNC, its pathogenesis and therapeutic approach. A total of 309 manuscripts were retrieved from electronic databases until December 01, 2021. After removing irrelevancy and duplicates, 61 manuscripts were taken for full-text screening, and, finally, 26 publications delivering outcomes of interest were included for review. Only one study peer-reviewed reporting ON/OP from this group was included [61]. No patient affected by NS and presenting ON/OP/EST/IDEMNC was found in this review. A PRISMA flow chart for the literature searched is shown below (Figure 1).
Only one male patient presented NS/ON/OP was identified through this searching research [61]. The aged of the patients was 51-year-old, but other clinical conditions such as ESTNC and SCNC were well ruled out. Therefore, our case is the first one affected by ON/OP/EST/IDEM/NS to be reported to the international medical community.
Case Presentation
A 37-year-old female patient complained of chronic headache, upper back pain, and progressive decreasing visual acuity since 2012, followed by mild gradual improvement until 2019 when she became worst and persistent amenorrhea was then present. The following year patient started to complain of nausea, vomiting, and her vision became cloudy more on the right side without gross improvement despite corticosteroid therapy. In May 2021, she was admitted to the ophthalmology ward because her visual acuity deteriorated, and she lost vision on the right eye three weeks after admission. She also complained of generalized intermittent headache and dizziness, not associated with other symptoms or signs, without aggravating or realizing factors but responding to routinary pain killers. HIV non-reactive with PCR for COVID-19 negative. The diagnosis of ON in the right eyes and optic peri-neuritis in the left eyes was made, and the patients were treated with IV methylprednisolone followed by oral prednisone 60 mg orally once a day 21 consecutive days after that patient was transferred to neurology awards. At the time of admission in neuro-wards patient complained of chronic upper back pain, painless blurred vision on the left eyes and complete blindness on the right eyes and bilateral weakness in the lower limbs, which progressed mildly, leading to mild gait instability over the last seven days before admission in neurology. She denied facial swelling or weakness, hearing disturbances, fever, skin rashes, or respiratory symptoms. No family history of any neurological disorders or granulomatous condition.
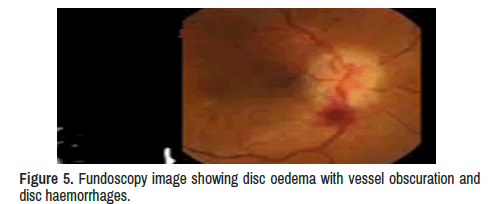
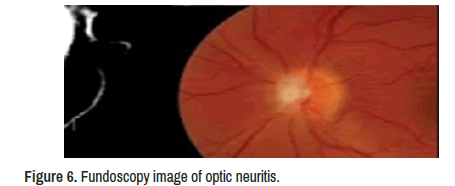
Neuro-ophthalmologic examination confirmed decreased visual acuity (20/160) in his left eye with decreased pupillary light reflex and mild disc oedema with vessel obscuration and disc haemorrhages. The visual acuity was absent on the right eyes, but specific ocular examination findings suggesting sarcoidosis, such as kerato precipitates, uveitis, conjunctivitis, or vascular sheathing in either eye were not reported, and only signs of optic neuritis (ON) were identified on the left eyes fundoscopy showed diffuse optic disc oedema, disc haemorrhages, vessel obscuration, and small flame-shaped retinal haemorrhages plus other signs of OP. In addition, Humphrey visual field testing confirmed complete visual field suppression in her right eye.
Laboratory test results
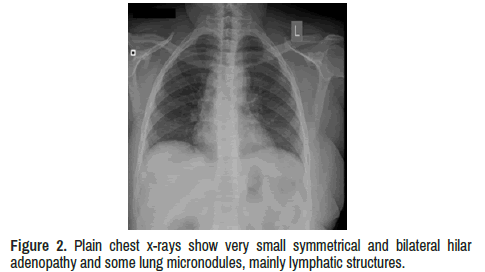
Chest x-ray shows symmetrical and bilateral hilar adenopathy and some lung micronodules, mainly lymphatic structures (Table 1 and Figure 2).
MRI brain and orbit: Technique-Axials-FLAIR, SWI, T1W+C, ADC, Coronals-T1W1, Sagittal-T1W.
Findings: No pre-septal swelling or mass lesions bilaterally.
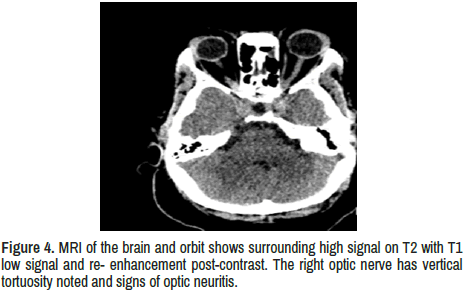
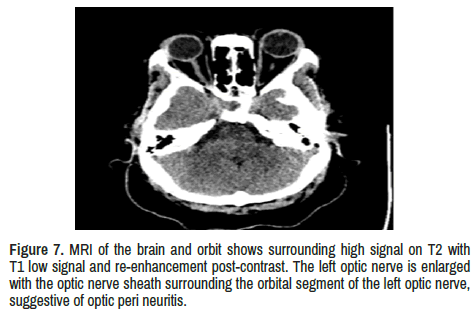
Optic nerves: bilateral high signal on T2 with T1 low signal and re- enhancement post-contrast. The right optic nerve has vertical tortuosity noted and signs of optic neuritis. In addition, the left optic nerve is enlarged with the optic nerve sheath surrounding the orbital segment of the left optic nerve, suggestive of optic peri neuritis.
Extraocular muscles are isodense to the soft tissue. No thickening of the extraocular muscles.
Retrobulbar fat normal. No masses in the retrobulbar region. There is no evidence of intraorbital mass or collection (Figures 2 and 3).
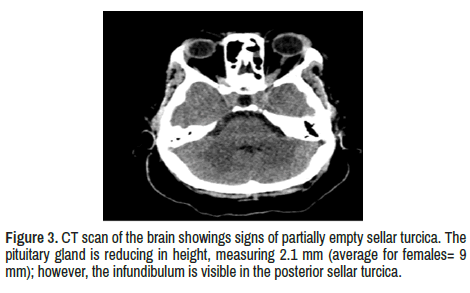
Bilateral orbital apices, Optic chiasma are normal. The pituitary gland is reducing height, measuring 2.1 mm (standard for females=9 mm); however, the infundibulum is visible in the posterior sellar turcica.
The visualized brain parenchymal is normal. No intra-or extra-axial focal lesions or masses. No areas of abnormal signal intensity. The ventricles are normal. No signs of Hydrocephalus. The brain stem and cerebellum are standard (Figures 4-7).
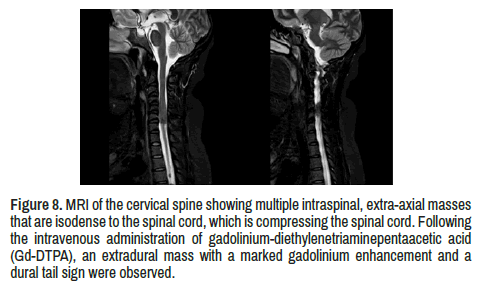
Visualized cervical spine multiple intraspinal, extra-axial masses that are isodense to the spinal cord, compressing the spinal cord. After intravenous administration of gadolinium-diethylenetriaminepentaacetic acid (Gd- DTPA), an extradural mass with a marked gadolinium enhancement and a dural tail sign were observed (Figure 8).
Right optic neuritis and left optic peri neuritis. Partially empty sellar turcica syndrome. Multiple cervical intra-thecal masses with cervical cord compression by probable neurosarcoidosis.
Figure 8. MRI of the cervical spine showing multiple intraspinal, extra-axial masses that are isodense to the spinal cord, which is compressing the spinal cord. Followingthe intravenous administration of gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), an extradural mass with a marked gadolinium enhancement and a dural tail sign were observed.
Results and Discussion
After a powerful searching of the literature, we did not find a case of NS combining ON/OP with empty sellar turcica syndrome and spinal tumour. Therefore, this patient is the first reported to the medical literature with such presentation from our knowledge.
Histopathology and differential diagnosis of sarcoidosis
After we searched the literature, we confirmed that histological patterns might differ according to the variants of Sa, including a mixture of a few necrotic tissues. In deep, the presence of loosely organized collections of mononuclear phagocytes/multinucleated giant cells, palisading granulomas, dirty necrosis, great surrounding inflammatory infiltrate (including lymphocytes, neutrophils, eosinophils, and plasma cells), extensive necrosis, few granulomas, lack of lymphatic distribution of granulomas, and secondary lymphoid follicles are not suggestive of Sa, and some authors found similar presentation in patients with berylliosis [60].
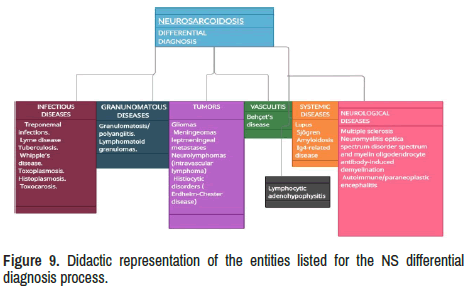
There is an extensive list of medical conditions to be considered during the differential diagnosis process. For didactic purposes and lack of space to discuss all of them one by one, we propose grouped in six categories such as (1) Infectious diseases, (2) Granulomatous diseases, (3) Benign tumour and malignancies, (4) Vasculitis, (5) Systemic diseases and (6) Neurological diseases have been represented in Figure 9.
Epidemiology and pathogenesis of neurosarcoidosis
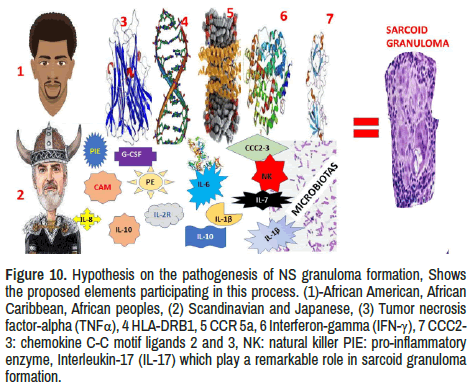
Although the aetiology of Sa remains unknown, some environmental features, microbes and occupational activities have been considered in its pathological process, including genetic disorders in some ethnicities. Inhalations of antigens may explain the initiation of the inflammatorygranulomatous process and the high frequency of respiratory and mediastinal lymph node involvement following to hyperinflammatory response caused by hyperproduction of Th1 cytokines like TNF-α, IFN-γ, IL- 1, among others. The participation of this inflammatory-mediated response mechanism in the production of granulomas has been documented by other authors [61-67]. Answering to our second research question, we have hypothesized the most probable elements involved in the pathogenesis of sarcoid granulomas formation, as is graphically represented in Figure 10.
Figure 10. Hypothesis on the pathogenesis of NS granuloma formation, Shows the proposed elements participating in this process. (1)-African American, AfricanCaribbean, African peoples, (2) Scandinavian and Japanese, (3) Tumor necrosis factor-alpha (TNFα), 4 HLA-DRB1, 5 CCR 5a, 6 Interferon-gamma (IFN-γ), 7 CCC2-3: chemokine C-C motif ligands 2 and 3, NK: natural killer PIE: pro-inflammatory enzyme, Interleukin-17 (IL-17) which play a remarkable role in sarcoid granuloma formation.
Sarcoidosis is an inflammatory pathology typifier by a heightened cell-mediated granulomatous response to yet unknown antigen (like other autoimmune diseases), but this hypothesis has been rejected by others [68,69].
Notwithstanding, we have hypothesized that some forms of NS are a direct consequence of hyperinflammatory state (HIS) with increased C reactive protein (CRP), INF 1-beta, TNF alpha and ferritin. Recently, we documented the relationship between HIS and subsequence activation of astrocytes and microglia injuring the CNS and the optic nerves and the SC [70-72].
Our case had elevated levels of CRP and ferritin, which support this hypothesis. Arguably, the presence of the HLA-DRB1*03 allele in some types of Lofgren syndrome (human leukocyte antigen) serve to support our proposal [73,74]. Based on results obtained from studies on pulmonary Sa, other authors have a similar opinion either [75].
Whilst our research community goes ahead looking for more secure information to demonstrate the relationship of NC with the immune system, on the other hand, other investigators continue working on genetic process related to NS. Some findings have proven that complex genetic types of inheritance associated high susceptible genes increase the risk of NC, and it is noticeable that monozygotic twins have more than 80 times chances to develop Sa than the average population; this risk is seven times higher in dizygotic twins and five times higher nontwin siblings. This susceptibility also depends on race and ethnicity features [76,77]. Nonetheless, there is no complete concordance between Sa siblings and outcome or phenotypes pattern.
Undoubtedly, some environmental factors may influence peoples from regions with high NS prevalence, like African American and Scandinavian peoples, graphically represented.
Both environmental antigens (infectious and non-infectious) facilitate the development of Sa granuloma [53].
The mechanism of inflammation and granuloma formation postulated in Figure 10 may clarify the neurological manifestations of NS secondary to the pathological disruption of the nervous system tissue microarchitecture caused by the accumulation of granulomas [76]. At this time, we like to highlight that Sa granulomas are composed of T helper cells within a ring of CD8+ cytotoxic, fibroblasts with scattered B cells and other proinflammatory cytokines, activated microglia and astrocytes supported by TNF-α, IFN-β/γ, IL-2, and CCC2-3: chemokine C-C motif ligands 2 and 3, among others which, through stimulating naive CD4+T cell produce and maintenance of Sa granulomas. In addition, it is essential to remember that IFN-β and IL-2 are produced by T helper cells, increasing the proliferation of immune cells plus cytotoxicity, and Th17-polarized effector T cells have been found in Sa granulomas which can modulate the NS outcome [77].
We have hypothesized that this hyperinflammatory response is caused by TNF-α, IFN-β/γ, IL-2, and CCC2-3: chemokine C-C motif ligands 2 and 3, plus HLA-DRB1, CCR 5a, pro-inflammatory enzyme (PIE), natural killer (NK), among other cause disruption of the average balance between firmicutes and bacteroidetes in the gut causing dysbiosis, neurohormonal dysfunction, neurotransmitter disturbances from the enteric nervous system causing damage on the CNS trough the gut-microbiota-brain axis and contribute to nervous system granuloma formation as is summarized in Figure 10. Another aspect to be considered in our case is the presence of sustainable amenorrhea related to pituitary damaged and favoured by the typical hypothalamus-pituitary-adrenal axis disturbance commonly caused by dysbiosis.
Disorder of the sella turcica
Around 10%-25% of patients with Sa complaint of Hypothalamic/ pituitary dysfunction mainly characterized by anterior hypopituitarism (ACTH 49%, GH 50%; TSH 67%, and LH/FSH 89%), diabetes insipidus (65%) and hyperprolactinemia (49%). Almost half of the patients with NS have a sellar turcica disease. As mentioned, our patient presented sustained amenorrhea, which did not improve despite corticosteroid therapy. Partially EST was confirmed by a CT scan of the brain (Figure 3). Some authors found thickening and gadolinium enhancement of the pituitary gland, or stalk with extension into the hypothalamus and even multifocal lesions in their MRI findings [6] Contrary, other investigators confirm that lesions on the Hypothalamus-pituitary region are an uncommon manifestation of Sa and they represent than 1% of all sellar turcica pathologies and when are present is almost always associated to sinonasal and neurological signs demanding systemic treatment [60].
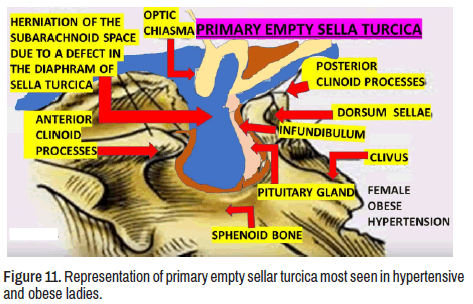
As we before mentioned, our patient has EST syndrome secondary to NS. We have hypothesized that the pathogenesis of primary EST considering the displacement and flattened off the hypophysis by herniated subarachnoid space (SAS) into the cavity of the sellar turcica is due to unspecified damage of the meningeal layer in the medial fossa at the base of the skull (Figures 11 and 12).
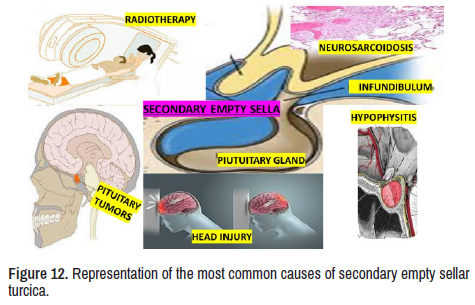
In Figure 12, several conditions have been listed as aetiology of secondary EST. Therefore, we will not discuss all of them due to space limitations. However, it is essential to highlight the mechanism of EST secondary to NS present in our report. Although the NS is a rarity affecting the subarachnoid membrane through granulomatous inflammatory injury, it is the only sustainable explanation for the herniation of SAS into the sella turcica cavity leading to pituitary damage by compression of the herniated bag of CSF clinically expressed as a pituitary disorder.
Conclusion
Unfortunately, to answer the first research question, we could not extensively search the whole medical literature, but from our comprehensive review, we concluded that our case is unique because we did not find a similar case.
Our review also concluded that Sa is an uncommon granulomatous disorder quite like many other infectious and autoimmune diseases. Therefore, soon after a confirmed diagnosis is made, a sustainable and dedicated long-term follow-up is mandatory to identify the best moment to initiate CS therapy with or without associated IA or others. We also agreed with those authors who recommended a multidisciplinary approach to afford neurological, cardiac, and ocular complications like our case.
To respond to our last research question, we delivered a personal hypothesis on the pathogenesis of the NS based on the role played by TNF-α, IFN-β/γ, and IL-2, dysbiosis, and other elements the mechanism of Sa granuloma formation.
Following editorial regulations, we must delete many aspects of this issue to reduce the length of this manuscripts.
Notwithstanding, large, and well-designed cross-section studies and RCT will be necessary to provide the best information about all this matter.
Declarations
Ethical considerations
This review was not considered ethical approval because all the data were extracted from previously published articles. Nevertheless, we obtained signed written consent from our patient for publishing her clinical information and images. Therefore, the Institutional Ethical Committee did not consider this study for additional ethical approval.
Competing interestThe author has not any conflict of interest to disclose. We declare no commercial or financial relationships construed as a potential conflict of interest.
FundingThe author declared that he never received any financial support or personal collaboration that could have influenced the results reported in this paper.
Declaration of anonymityThe author certifies that he did not reveal the names, surnames, initials. Alternatively, other identity issues of this case in this publication and complete anonymity are guaranteed.
Availability of data and material: The data that support the findings of this study are available on reasonable request from the corresponding author.
Acknowledgement
We sincerely thank Dr Lourdes de Fátima Ibañez Valdés for her enthusiastic support and management of this patient.
References
- Lynch III, Joseph P, Ella A Kazerooni and Steven E. Gay. "Pulmonary Sarcoidosis." Clin Chest Med 18 (1997): 755-785.
[Crossref]
- MacLean, Heather J, and Mohammad Abdoli. "Neurosarcoidosis as an MS Mimic: The Trials and Tribulations of Making a Diagnosis." Mult Scler Relat Disord 4 (2015): 414-429.
- Belperio, John A, Faisal Shaikh, Fereidoun Abtin and Michael C. Fishbein, et al. "Extrapulmonary Sarcoidosis with a Focus on Cardiac, Nervous System, and Ocular Involvement." EClinicalMedicine 37 (2021): 100966.
- Culver, Daniel A, Manuel L Ribeiro Neto, Brandon P Moss, and Mary A Willis. "Neurosarcoidosis." Thieme Medical 38 (2017): 499-513.
- Carlson, Matthew L, James R White Jr, Mana Espahbodi, and David S Haynes, et al. "Cranial Base Manifestations of Neurosarcoidosis: A Review of 305 Patients." Otol Neurotol 36 (2015): 156-166.
- Langrand, C, H Bihan, G Raverot, and L Varron, et al. "Hypothalamo-Pituitary Sarcoidosis: A Multicenter Study of 24 Patients." QJM-INT J MED 105 (2012): 981-995.
- Leonhard, Sonja E, Daan Fritz, Filip Eftimov, and Anneke J van der Kooi, et al. "Neurosarcoidosis in a Tertiary Referral Center: A Cross-Sectional Cohort Study." Medicine 95 (2016): e3277.
- Aubart, Fleur Cohen, Diane Bouvry, Damien Galanaud, and Caroline Dehais, et al. "Long-Term Outcomes of Refractory Neurosarcoidosis Treated with Infliximab." J Neurol 264 (2017): 891-897.
- Judson, Marc A, Robert P Baughman, Bruce W Thompson, and Alvin S Teirstein, et al. "Two Year Prognosis of Sarcoidosis: The ACCESS Experience." Sarcoidosis Vasc Diffuse Lung Dis 20 (2003): 204-211.
- Iwai, Kazuro, Teruo Tachibana, Tamiko Takemura, and Yasuo Matsui, et al. "Pathological Studies on Sarcoidosis Autopsy. I. Epidemiological Features of 320 Cases in Japan." Pathol Int 43 (1993): 372-376.
- Dorman, James, Lakshmi Warrior, Vishal Pandya, and Ying Sun, et al. "Neurosarcoidosis in a Public Safety Net Hospital: A Study of 82 Cases." Neurology (2017): 389.
- Kreider, Mary Elizabeth, Jason D Christie, Bruce Thompson, and Lee Newman, et al. "Relationship of Environmental Exposures to the Clinical Phenotype of Sarcoidosis." Chest 128 (2005): 207-215.
- Rybicki, Benjamin A, Marcie Major, John Popovich Jr, and Mary J Maliank, et al. "Racial Differences in Sarcoidosis Incidence: A 5-Year Study in a Health Maintenance Organization." Am J Epidemiol 145 (1997): 234-241.
- Semenzato, Gianpietro. "ACCESS: A Case Control Etiologic Study of Sarcoidosis." Sarcoidosis Vasc Diffuse Lung Dis 22 (2005): 83-86.
- Freemer, Michelle, and Talmadge E King Jr. "The ACCESS Study: Characterization of Sarcoidosis in the United States." Am J Respir Crit Care Med 164 (2001): 1754-1755.
- Sohn, Mimi, Kenkichi Nozaki, Daniel A Culver, and Marc A Judson, et al. "Spinal Cord Neurosarcoidosis." Am J Med Sci 347 (2014): 195-198.
[Crossref]
- Wengert, Oliver, Eva Rothenfusser-Korber, Boris Vollrath, and Georg Bohner, et al. "Neurosarcoidosis: Correlation of Cerebrospinal Fluid Findings with Diffuse Leptomeningeal Gadolinium Enhancement on MRI and Clinical Disease Activity." J Neurol Sci 335 (2013): 124-130.
- Gelfand, Jeffrey M, Michael J Bradshaw, Barney J Stern, and David B Clifford, et al. "Infliximab for the Treatment of CNS Sarcoidosis: A Multi-Institutional Series." Neurology 89 (2017): 2092-2100.
- Metyas, Samy, Magdy Tawadrous, Karen C Yeter, and Daniel G Arkfeld. "Neurosarcoidosis Mimicking Multiple Sclerosis Successfully Treated with Methotrexate and Adalimumab." Int J Rheum Dis 17 (2013): 214-216.
- Marnane, Michael, Timothy Lynch, Jaqueline Scott, and John Stack, et al. "Steroid-Unresponsive Neurosarcoidosis Successfully Treated with Adalimumab." J Neurol 256 (2009): 139.
- Padooru, Keerthi Reddy, and Mitali Sen. "Intramedullary Spinal Cord Involvement: A Rare Presentation of Sarcoidosis." Int Med Case Rep J 12 (2019): 199.
- Longo, Michael, Yaroslav Gelfand, Merritt D Kinon, and James Pullman, et al. "Multifocal Epidural Neurosarcoidosis Causing Spinal Cord Compression: A Case Report." Cureus 11 (2019):19-21.
- Gastaldi, Matteo, Enrico Marchioni, Paola Banfi, and Valeria Mariani, et al. "Predictors of Outcome in a Large Retrospective Cohort of Patients with Transverse Myelitis." Mult Scler J 24 (2018): 1743-1752.
- Scott, Amanda Mary, Janeth Yinh, Timothy McAlindon, and Robert Kalish. "Two Cases of Sarcoidosis Presenting as Longitudinally Extensive Transverse Myelitis." Clin Rheumatol 37 (2018): 2899-2905.
- Sohn, Mimi, Culver DA, Judson MA, and Scott TF, et al. “Spinal Cord Neurosarcoidosis.” Am J Med Sci. 347:195–198 (2014).
[Crossref]
- Wang, Lei, and Yuebing Li. "Longitudinal Ultra-Extensive Transverse Myelitis as a Manifestation of Neurosarcoidosis.” J Neurol Sci 355 (2015): 64-67.
- Bathla, G, AK Singh, B Policeni, and A Agarwal, et al. "Imaging of Neurosarcoidosis: Common, Uncommon, and Rare." Clin Radiol 71 (2016): 96-106.
- Bose, Bikash. "Extramedullary Sarcoid Lesion Mimicking Intraspinal Tumor." Spine J 2 (2002): 381-385.
- Schaller, Bernhard, Thomas Kruschat, Holger Schmidt, and Wolfgang Brück, et al. "Intradural, Extramedullary Spinal Sarcoidosis: Report of a Rare Case and Review of the Literature." Spine J 6 (2006): 204-210.
- Hoyle, J Chad, Courtney Jablonski, and Herbert B Newton. "Neurosarcoidosis: Clinical Review of a Disorder with Challenging Inpatient Presentations and Diagnostic Considerations." Neurohospitalist 4 (2014): 94-101.
- Galgano, Michael A, Carlos R Goulart, Karen Chisholm, and Melissa Hazen, et al. "Rapid-Onset Thoracic Myelopathy due to an Epidural Sarcoid-like Lesion in a Pediatric Patient." World Neurosurg 111 (2018): 377-380.
- Weissman, Mark N, Ross Lange, Chris Kelley, and Kathy Belgea, et al. "Intraspinal Epidural Sarcoidosis: Case Report." Neurosurgery 39 (1996): 179-181.
- Nardone, Raffaele, Alessandro Venturi, Ebba Buffone, and Piergiorgio Lochner, et al. "Extramedullary Spinal Neurosarcoidosis: Report of Two Cases." Eur Neurol 54 (2005): 220.
- Barazi, S, I Bodi, and N Thomas. "Sarcoidosis presenting as an Isolated Extradural Thoracolumbar Lesion Mimicking Tumour." Br J Neurosurg 22 (2008): 690-691.
- Joubert, Bastien, Catherine Chapelon-Abric, Lucie Biard, and David Saadoun, et al. "Association of Prognostic Factors and Immunosuppressive Treatment with Long-Term Outcomes in Neurosarcoidosis." JAMA Neurol 74 (2017): 1336-1344.
- Bradshaw, Michael J, Siddharama Pawate, Laura L Koth, and Tracey A Cho, et al. "Neurosarcoidosis: Pathophysiology, Diagnosis, and Treatment." Neurol Neuroimmunol Neuroinflamm 8 (2021): e1084.
- Durel, Cécile-Audrey, Romain Marignier, Delphine Maucort-Boulch, and Jean Iwaz, et al. "Clinical Features and Prognostic Factors of Spinal Cord Sarcoidosis: A Multicenter Observational Study of 20 BIOPSY-PROVEN patients." J Neurol 263 (2016): 981-990.
- Soni, Neetu, Girish Bathla, and Ravishankar Pillenahalli Maheshwarappa. "Imaging Findings in Spinal Sarcoidosis: A Report of 18 Cases and Review of the Current Literature." Neuroradiol J 32 (2019): 17-28.
- Flanagan, Eoin P, Timothy J Kaufmann, Karl N Krecke, and Allen J. Aksamit, et al. "Discriminating Long Myelitis of Neuromyelitis Optica from Sarcoidosis." Ann Neurol 79 (2016): 437-447.
- Zalewski, Nicholas L, Karl N. Krecke, Brian G Weinshenker, and Allen J Aksamit, et al. "Central Canal Enhancement and the Trident Sign in Spinal Cord Sarcoidosis." Neurology 87 (2016): 743-744.
- Murphy, Olwen C, Salazar-Camelo A, Jimenez J, and Barreras P, et al. “Clinical and MRI Phenotypes of Sarcoidosis-Associated Myelopathy.” Neurol Neuroimmunol Neuroinflamm. 7(2):e722 (2020).
- Ishiwata, Sho, Yoichi Iizuka, Tokue Mieda, and Junko Hirato, et al. "Intradural Extramedullary Spinal Sarcoidosis Mimicking Meningioma." Case Rep Orthop 2019 (2019).
- Hamasaki, Takahiko, Masayuki Noda, Naosuke Kamei, and Susumu Yamamoto, et al. “Intradural Extramedullary Mass Formation in Spinal Cord Sarcoidosis: Case Report and Literature Review.” Spine 28 (2003): E420–E423.
- Roy, Kaushik, P Tripathy, A Senapati, and SK Saha. "Intradural Extramedullary Sarcoidosis Case Report and Review of Literature." Asian J Neurosurg 5 (2010): 87.
- Fazlullah, S. "Sarcoidosis with Involvement of the Nervous System." Diseases 41 (1962): 685-688.
- Day, Arthur L, and George W Sypert. "Spinal Cord Sarcoidosis." Ann Neurol 1 (1977): 79-85.
- Baruah, Jitendra K, Franz E Glasauer, Ranajit Sil, and Bernard H Smith. "Sarcoidosis of the Cervical Spinal Canal: Case Report." Neurosurgery 3 (1978): 216-2186.
- Christoforidis, Greg A, Eric M Spickler, Maria V Recio, and Bharat M Mehta. "MR of CNS Sarcoidosis: Correlation of Imaging Features to Clinical Symptoms and Response to Treatment." Am J Neuroradiol 20 (1999): 655-669.
- Connor, SE J, L Marshman, S Al-Sarraj, and V Ng. "MRI of a Spinal Intradural Extramedullary Sarcoid Mass: A Case Report and Literature Review." Neuroradiology 43 (2001): 1079-1083.
- Bose, Bikash. Extramedullary Sarcoid lesion Mimicking Intraspinal Tumor. Spine J. 2(5):381–385 (2002).
- Kleinschmidt-Demasters, BK, David Ormond, and Evan Winograd. "NIMG-61. Unusual Presentation of Neurosarcoidosis: A Case Series." Neuro Oncol 22 (2020): 161.
- Asranna, Ajay, Aneesh Mohimen, Roopa Rajan, and Muralidharan Nair. "Neurosarcoidosis as a Cause of Longitudinally Extensive Myelitis: Neuroimaging Clues for Diagnosis." Ann Indian Acad Neurol 22 (2019): 100. [Crossref] [Google Scholar]
- Scott, Thomas F, Kristin Yandora, April Valeri, and Carol Chieffe, et al. "Aggressive Therapy for Neurosarcoidosis: Long-Term Follow-Up of 48 Treated Patients." Arch Neurol 64 (2007): 691-696.
- Bogousslavsky, J, JP Hungerbühler, F Regli, and HJ Graf. "Subacute Myelopathy as the Presenting Manifestation of Sarcoidosis." Acta Neurochir 65 (1982): 193-197.
- Spencer, Terri S, Joseph V Campellone, Irama Maldonado, and Ning Huang, et al. "Clinical and Magnetic Resonance Imaging Manifestations of Neurosarcoidosis." Semin Arthritis Rheum 34(2005): 649-661.
- Sève, Pascal, Yves Pacheco, François Durupt, and Yvan Jamilloux, et al. "Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis." Cells 10 (2021): 766.
- Lin, Mung Yan, Qun Wang, Nancy J Newman, and Michael Dattilo. "An Unusual Presentation of Neurosarcoidosis: Concurrent Optic Perineuritis and Optic Neuritis." Taiwan J Ophthalmol 11 (2021): 104.
- Busuttil, A, SS Weigt, MP Keane, and YY Xue, et al. "CXCR3 Ligands are Augmented during the Pathogenesis of Pulmonary Sarcoidosis." Eur Respir J 34 (2009): 676-686.
- Palchevskiy, Vyacheslav, Nastran Hashemi, Stephen S Weigt, and Ying Ying Xue, et al. "Immune Response CC Chemokines CCL2 and CCL5 are Associated with Pulmonary Sarcoidosis." Fibrogenesis Tissue Repair 4 (2011): 1-12.
- Gaddam, Mrunanjali, Ugochi Ojinnaka, Zubayer Ahmed, and Amudhan Kannan, et al. "Sarcoidosis: Various Presentations, Coexisting Diseases and Malignancies." Cureus 13 (2021): e16967.
- Weigt, S Samuel, Vyacheslav Palchevskiy, and John A Belperio. "Inflammasomes and IL-1 Biology in the Pathogenesis of Allograft Dysfunction." J Clin Invest 127 (2017): 2022-2029.
- Kron, Jordana, Adolfo Gabriele Mauro, Aldo Bonaventura, and Stefano Toldo, et al. "Inflammasome Formation in Granulomas in Cardiac Sarcoidosis." Circ Arrhythm Electrophysiol 12 (2019): e007582.
- Huppertz, Christine, Benedikt Jäger, Grazyna Wieczorek, and Peggy Engelhard, et al. "The NLRP3 inflammasome pathway is activated in sarcoidosis and involved in granuloma formation." Eur Respir J 55 (2020).
- Moller, David R, Ben A Rybicki, Nabeel Y Hamzeh, and Courtney G Montgomery, et al. "Genetic, Immunologic, and Environmental Basis of Sarcoidosis." Ann Am Thorac Soc 14 (2017): S429-S436.
- Van Moorsel, Coline HM, and David C. Christiani. "Genetic Susceptibility to Sarcoidosis, a Chronic Inflammatory Disorder." Am J Respir Crit Care Med 186 (2012): 816-818.
- Sibat, Humberto Foyaca. "Comorbidity of Neurocysticercosis, HIV, Cerebellar Atrophy and SARS-CoV-2: Case Report and Systematic Review." Clin Schizophr Relat Psychoses 15 (2022): 1-6.
- Sibat, Humberto Foyaca. "Neurocysticercosis, Epilepsy, COVID-19 and a Novel Hypothesis: Cases Series and Systematic Review." Clin Schizophr Relat Psychoses 15 (2021): 1-13.
- Sibat, Humberto Foyaca. "Intracranial Hypotension after Severe COVID-19: Case Report and Literature Review." Clin Schizophr Relat Psychoses 15 (2021): 1-11.
- Berlin, Mary, Anna Fogdell-Hahn, Olle Olerup, and Anders Eklund, et al. "HLA-DR Predicts the Prognosis in Scandinavian Patients with Pulmonary Sarcoidosis." Am J Respir Crit Care Med 156 (1997): 1601-1605.
- Grunewald, Johan, and Anders Eklund. "Lofgren's Syndrome: Human Leukocyte Antigen Strongly Influences the Disease Course." Am J Respir Crit Care Med 179 (2009): 307-312.
- Rose, Noel R, and Constantin Bona. "Defining Criteria for Autoimmune Diseases (Witebsky's Postulates Revisited)." Immunol Today 14 (1993): 426-430.
- Fingerlin, Tasha E, Nabeel Hamzeh, and Lisa A Maier. "Genetics of Sarcoidosis." Clin Chest Med 36 (2015): 569-584.
- Judson, Marc A, Kathryn Hirst, Sudha K Iyengar, and Benjamin A Rybicki, et al. "Comparison of Sarcoidosis Phenotypes among Affected African-American siblings." Chest 130 (2006): 855-862.
- Iannuzzi, Michael C, and Joseph R Fontana. "Sarcoidosis: Clinical Presentation, Immunopathogenesis, and Therapeutics." JAMA 305 (2011): 391-399.
- Baughman, Robert P, SA Strohofer, J Buchsbaum, and EE Lower. "Release of Tumor Necrosis Factor by Alveolar Macrophages of Patients with Sarcoidosis." J Lab Clin Med 115 (1990): 36-42.
- Anthony, Jeremy, Gregory J Esper, and Adriana Ioachimescu. "Hypothalamic–Pituitary Sarcoidosis with Vision Loss and Hypopituitarism: Case Series and Literature Review." Pituitary 19 (2016): 19-29.
- Langrand, C, Bihan H, and Raverot G, et al. Hypothalamo-pituitary sarcoidosis: a multicenter study of 24 patients. QJM. 105(10):981-995 (2012).
Citation: Foyaca-Sibat, Humberto. "Unique Presentation of Neurosarcoidosis: Case Report and Systematic Review”. Clin Schizophr Relat Psychoses 16S (2022). Doi: 10.3371/CSRP.SF.021022
Copyright: © 2022 Foyaca-Sibat H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.