Brief Report - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
The Mitochondrial Dna Dloop Variations In Depressed Patients
Alaa Ibraheem Lazim1, Hajer Ali Enad2, Ahmed Khalid Aldhalmi3, Albab Fawwaz Alfarras4, Hawraa Fadhel Abbas AL-Baghdady5, Noora M Hameed6*, Sundus Mohammad Ali Al-Bazi7 and Ali Jalil Obaid82Department of Medical Laboratory Techniques, AL‐Mustaqbal University, Hillah, Iraq
3Department of Medical Laboratory Techniques, The University of Mashreq, Baghdad, Iraq
4Department of Medical Laboratory Techniques, Al-Farahidi University, Baghdad, Iraq
5Department of Medical Laboratory Techniques, The Islamic University, Najaf, Iraq
6Department of Medical Laboratory Techniques, Al-Nisour University, Baghdad, Iraq
7Department of Medical Laboratory Techniques, Al‐Zahrawi University, Karbala, Iraq
8Department of Medical Laboratory Techniques, Hilla University, Babylon, Iraq
Noora M Hameed, Department of Medical Laboratory Techniques, Al-Nisour University, Baghdad, Iraq, Email: noora.anesth@nuc.edu.iq
Received: 08-Nov-2021 Accepted Date: Nov 22, 2021 ; Published: 29-Nov-2021, DOI: 10.3371/CSRP.AMHE.091221
Abstract
Depression is one of an important health problems in the last years, the predisposition to the depression still under investigations, the present study deal with the mtDNA variation loci in depression patients compare with control, PCR-sequencing was used to determine mutations, results show that there were among 173 nucleotides of the D-loop (16034-16207) there were 19 sites differentiated in non-significant differences, about 8 types of substitution mutations were observed included (A>C, A>G, T>C, G>A, C>T, C>A, C>G, G>A), insertion and deletion mutations also founded, non-significant differences were appeared in the types and number of mutations in this segment of mtDNA. The present output concluded that there wasn’t association between D-loop variations and depression disease.
Keywords
Mitochondrial DNA • D-loop • Variations • Depression patients
Introduction
The Mitochondria organelle is playing a critical role in energy production, lipid and steroid metabolism, and cellular stability, as well as modulating Ca2+ levels, maintaining ROS levels, and regulating apoptosis [1]. As a result, mitochondrial dysfunction not only makes it difficult for cells to get their energy needs, but it may also be contributed in the neuronal communication and cellular signals impairment [2].
The notion of neuroplasticity is at the center of a new mood disorder theory, which focuses on bipolar disorder and depression. The term "neuroplasticity" refers to the brain's malleability, which includes synaptic and non-synaptic plasticity; Synaptic plasticity is the process of neurons responding to changes in their exogenous and endogenous environment by generating alterations in brain pathways and synapses. It includes synapticgenesis, axon and dendritic development, and the elimination of superfluous linkage between neurons. Mitochondria play a crucial role in neuroplasticity, and it has been well established that stress causes structural and functional damage in many brain areas of individuals suffered from depression, resulting in decreased neuroplasticity [3].
The Major Depressive Disorder (MDD) is a prevalent mental condition [4]. Some investigations suggested that the neurotropic factors, neuroplasticity, and mitochondrial dysfunction for biological hypotheses proposed of MDD because the MDD pathophysiology still under studying [5]. the impairment of Mitochondrial functions was affecting in cellular processes due to impairments in cellular resilience and synaptic plasticity [6], and has also been associated with psychiatric illnesses, such as bipolar disorder, MDD, anxiety disorder and schizophrenia [7,8].
Several investigations have shown a connection of depression with mitochondrial malfunction, and systematic psychiatric assessments of individuals with mitochondrial illness have revealed that around half of these patients had a lifetime MDD prevalence [9,10]. Measuring mtDNA copy numbers can be used to measure mitochondrial function indirectly. Cells that require a lot of energy, including heart cells, skeletal muscle cells, and neurons, need a lot of ATPS and have a lot of mtDNA copies, which is considered a measure of mitochondrial energy function [11]. The presence of abnormal mtDNA copy numbers has been linked to a variety of mental illnesses [12].
Materials and Methods
Sample collection
A 24 depression, blood samples were collected from (Margan medical city Hospital, Babylon–Iraq), which were diagnosed by specialist, physician prof. Dr. kareem Naser, and 20 blood samples of control were collected from healthy people, their age ranged (23 years to 70 years).
Genomic DNA extraction
DNA was isolated from whole blood for both groups using a DNA extraction kit (Favorgen), and then DNA concentrations and purity were estimated using a Nanodrop.
The primer of HV1a gene
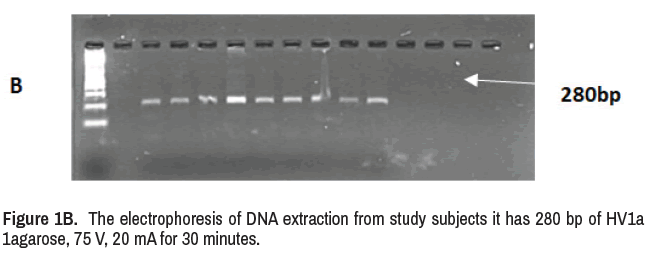
It was used Forward 5- CAC CAT TAG CAC CCA AAG CT-3, Reverse 5- GGC TTT GGA GTT GCA GTT GAT -3 the PCR amplicon size (280 bp) with annealing temperature reached to 58.8ºC The products visualized by electrophoresis (1agarose, 75 V, 20 mA for 40 min).
Capillary electrophoresis
The capillary electrophoresis was carried out using genetic analyzer (applied bio system 3600), data were interpretated using software, then analysis by NCBI blast, https://blast.ncbi.nlm.nih.gov/Blast.cgi, sms bioinformatics software http://www.bioinformatics.org/sms2/pcr_products. html, and MAFFT version 7 https://mafft.cbrc.jp/alignment/server/. In comparison with (15978- 16257 in Homo sapiens mitochondrion).
Results
Twenty-four patients were diagnosed with major depression by physician, The age of the patients (16 females and 8 males) was in the range of 23 years to 70 years, and twenty samples as control group age ranged (20 years to 62 years, DNA was extracted from the whole blood of patients and control, its concentration was (50 ng/μl to 150 ng/μl) and purity was (1.8-2.1, The results of DNA extraction and PCR products (280 bp) were shown in Figures 1A and 1B, PCR product were identical with virtual amplifications which used latter in multiple comparison.
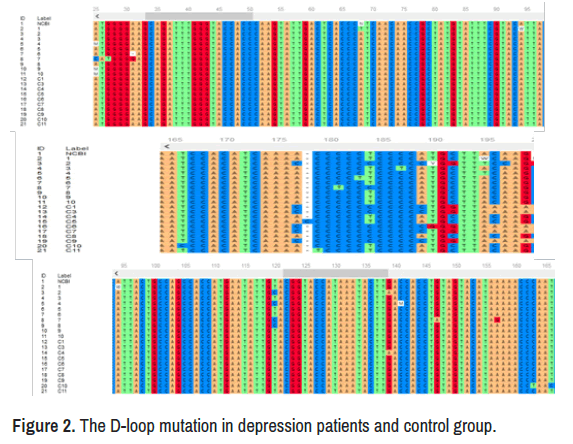
The mtDNA sequences show different mutations in study groups in different sites, there were among 173 nucleotides of the D-loop (16034- 16207) as showed in Table 1, there were 19 sites differentiated in non- significant differences, about 8 types of substitutions mutation were observed included (A>C, A>G, T>C, G>A, C>T, C>A, C>G, G>A), insertion and deletion mutations also founded, non-significant differences were appeared in the types and number of mutations in this segment of mtDNA as showed in Table 2 and Figure 2.
| mtDNA loci | NCBI | Patients (%) | Control | Odd ratio (CI%) | Sig |
|---|---|---|---|---|---|
| 16034 | A | C (10%) | A(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16040 | A | Deletion (10%) G (10%) |
A (100%) A(100%) |
3.3158 (0.1200 to 91.607) 3.3158 (0.1200 to 91.607) |
0.4790 0.4790 |
| 16077 | N | T(10%) | A(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16129 | T | C(30%) | T(100%) | 9.8000 (0.4380 to 219.259) |
0.1500 |
| 16148 | G | A(10%) | A(10%) | 1.0000 (0.0538 to 18.574) |
1 |
| 16156 | G | A(10%) | G(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16166 | A | G(10%) | A(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16172 | C | C | T(10%) | 0.3016 (0.0109 to 8.3321) |
0.4790 |
| 16175 | T | T | C(10%) | 0.3016 (0.0109 to 8.3321) |
0.4790 |
| 16186 | A | C(10%) | C(50%) | 9.0000 (0.8088 to 100.143) |
0.0739 |
| 16187 | - | - | Insertion | 133.00 (4.8140 to 3674.45) |
0.0039* |
| 16188 | C | T(10%) | C(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16191 | C | T(10%) | C(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16193 | A | C(10%) | C(50%) | 0.1111 (0.0100 to 1.236) |
0.0739 |
| 16201 | C | G(10%) | G(50%) | 0.1111 (0.0100 to 1.236) |
0.0739 |
| 16204 | A | T(20%) | A(100%) | 6.1765 (0.2599 to 146.78) |
0.2600 |
| 16205 | C | C | A(50%) | 0.0476 0.0022 to 1.0293 |
0.0522 |
| 16206 | A | C(10%) | A(100%) | 3.3158 (0.1200 to 91.607) |
0.4790 |
| 16207 | G | G | A(50%) | 0.0476 0.0022 to 1.0293 |
0.0522 |
| Note: *Significance< 0.005 | |||||
| Mutation types | P | C | Odd ratio (CI 95%) | Sig 0.005 |
|---|---|---|---|---|
| A>C | 3 | 2 | 1.7143 (0.2192 to 13.407) |
0.607 |
| A>G | 2 | 0 | 6.1765 (0.2599 to 146.785) |
0.2600 |
| T>C | 1 | 0 | 3.3158 (0.1200 to 91.607) |
0.479 |
| G>A | 2 | 1 | 2.2500 (0.1701 to 29.768) |
0.5383 |
| C>T | 2 | 1 | 2.2500 (0.1701 to 29.768) |
0.5383 |
| C>A | 0 | 1 | 0.3016 (0.0109 to 8.332) |
0.4790 |
| C>G | 1 | 1 | 1 (0.0546 to 18.30) |
1 |
| G>A | 2 | 2 | 1 (0.1118 to 8.9473)1 |
1 |
| Deletion | 2 | 0 | 6.1765 (0.2599 to 146.785) |
0.2600 |
| Insertion | 0 | 1 | 0.3016 (0.0109 to 8.332) |
0.4790 |
| Total | 15 | 9 | 1.7300 (0.7359 to 4.0670) |
0.2088 |
| 173 | 173 |
Discussion
Mitochondria have bioenergetics role in cells, furthermore any mutation or DNA damage in DNA can be led to some disease, and abnormalities in mitochondrial DNA have a key role in neurodegenerative disorders [13]. Interestingly, the accumulation of mitochondrial nucleotide changes and the instability of mtDNA have been linked to a variety of illnesses, including cancer and neurological disorders. The D-loop area is crucial in mitochondrial genome replication, transcription, and organization, and mutations in this region have been linked to mitochondrial genome instability [14].
The result of this study agreement with a study conducted by Munakata, et al. that explain that there is no associated between depression and mDNA mutation and this study explain that depression are caused by mutation in mDNA , in contrast with current study [15], several studies showed that Patients with MDD exhibited a greater mtDNA copy number than control individuals, and these findings were consistent whether the patient experienced a single episode or recurring MDD [4]. The reasons for the inconsistencies in previous findings of mtDNA copy number of patients with depression remain unclear. Some studies have reported higher mtDNA copy numbers in MDD cohorts [16,17], while others have reported lower mtDNA copy numbers in individuals with depression, or no changes in mtDNA copy number [18,19].
The discrepancies might be attributed to variation in individuals' medication status, disease duration, study population age compositions (young vs. elderly), challenging to homogenize phenotypes of diverse MDD diagnoses, and/or comorbidity of somatic disorders [20,21].
As a result of Reactive Oxygen Species (ROS) elevation level, which production in the organelle and the low level of Mismatch Repair (MMR) the DNA of mitochondria is highly susceptible to mutations, Although mutations observed throughout the mitochondrial genome, the D-loop is the most variable part of the human mitochondrial genome in addition to other mutations in different sites of genome, So we need to more study and whole mtDNA sequencing to more demonstrations of variations associated with major depression [22-24].
Conclusion
The current study found that the depression disorder didn’t relate to HV1 variation in compare with control group, So we need more studies and whole mtDNA sequencing to more demonstrations of variations associated with major depression disorder. The Mitochondria organelle is important for energy production, lipid and steroid metabolism, and cellular stability, as well as modulating Ca2+ levels, maintaining ROS levels, and controlling apoptosis.
References
- Scheffler, Immo E. Mitochondria. New Jersey: Wiley, USA, (2011).
- Rezin, Gislaine T., Cinara L. Gonçalves, Juliana F. Daufenbach and Daiane B. Fraga, et al. "Acute Administration of Ketamine Reverses the Inhibition of Mitochondrial Respiratory Chain Induced by Chronic Mild Stress." Brain Res Bull 79 (2009): 418-21.
- Sheline, Yvette I., Mokhtar H. Gado and Helena C. Kraemer. "Untreated Depression and Hippocampal Volume Loss." Am J Psychiatry 160 (2003): 1516-8.
- Chung, Jae Kyung, Soo Young Lee, Mira Park and Eun-Jeong Joo, et al. "Investigation of Mitochondrial DNA Copy Number in Patients with Major Depressive Disorder." Psychiatry Res 282 (2019): 112616.
- Manji, Husseini, Tadafumi Kato, Nicholas A. Di Prospero and Seth Ness, et al. "Impaired Mitochondrial Function in Psychiatric Disorders." Nat Rev Neurosci 13 (2012): 293-307.
- Schloesser, Robert J., Husseini K. Manji and Keri Martinowich. "Suppression of Adult Neurogenesis Leads to an Increased HPA Axis Response." Neuroreport 20 (2009): 553.
- Jou, Shaw-Hwa, Nan-Yin Chiu and Chin-San Liu. "Mitochondrial Dysfunction and Psychiatric Disorders." Chang Gung Med J 32 (2009): 370-9.
- Schapira, Anthony HV. "Mitochondrial Disease." Lancet 368 (2006): 70-82.
- Inczedy-Farkas, Gabriella, Viktoria Remenyi, Aniko Gal and Zsofia Varga, et al. "Psychiatric Symptoms of Patients with Primary Mitochondrial DNA Disorders." Behav Brain Funct 8 (2012): 1-9.
- Mancuso, Michelangelo, Daniele Orsucci, Elena Caldarazzo Ienco and Eleonora Pini, et al. "Psychiatric Involvement in Adult Patients with Mitochondrial Disease." Neurol Sci 34 (2013): 71-74.
- Montier, Laura L. Clay, Janice J. Deng and Yidong Bai. "Number Matters: Control of Mammalian Mitochondrial DNA Copy Number." J Genet Genomics 36 (2009): 125-31.
- Yoo, Hee Jeong, Mira Park and Soon Ae Kim. "Difference in Mitochondrial DNA Copy Number in Peripheral Blood Cells between Probands with Autism Spectrum Disorders and their Unaffected Siblings." World J Biol Psychiatry 18 (2017): 151-6.
- Mancuso, Michelangelo, Fabio Coppede, Lucia Migliore and Gabriele Siciliano, et al. "Mitochondrial Dysfunction, Oxidative Stress and Neurodegeneration." J Alzheimers Dis 10 (2006): 59-73.
- Ferreira, Ildete L., Maria V. Nascimento, Márcio Ribeiro and Sandra Almeida, et al. "Mitochondrial-Dependent Apoptosis in Huntington's Disease Human Cybrids." Exp Neurol 222 (2010): 243-55.
- Munakata, Kae, Kumiko Fujii, Shinichiro Nanko and Hiroshi Kunugi, et al. "Sequence and Functional Analyses of mtDNA in a Maternally Inherited Family with Bipolar Disorder and Depression." Mutat Res 617 (2007): 119-24.
- Nicod, Jérôme, Stefanie Wagner, Frederick Vonberg and Amarjit Bhomra, et al. "The Amount of Mitochondrial DNA in Blood Reflects the Course of a Depressive Episode." Biol Psychiatry 80 (2016): e41-e42.
- Tyrka, Audrey R., Stephanie H. Parade, Lawrence H. Price and Hung-Teh Kao, et al. "Alterations of Mitochondrial DNA Copy Number and Telomere Length with Early Adversity and Psychopathology." Biol Psychiatry 79 (2016): 78-86.
- Chang, Cheng-Chen, Shaw-Hwa Jou, Ta-Tsung Lin and Te-Jen Lai, et al. "Mitochondria DNA Change and Oxidative Damage in Clinically Stable Patients with Major Depressive Disorder." PLoS One 10 (2015): e0125855.
- Kim, Moo-Young, Ji-Won Lee, Hee-Cheol Kang and Eosu Kim, et al. "Leukocyte Mitochondrial DNA (mtDNA) Content is Associated with Depression in Old Women." Arch Gerontol Geriatr 53 (2011): e218-e221.
- He, Ying, Jinsong Tang, Zongchang Li and Hong Li, et al. "Leukocyte Mitochondrial DNA Copy Number in Blood is not associated with Major Depressive Disorder in Young Adults." PLoS One 9 (2014): e96869.
- Verhoeven, J. E., D. Révész, M. Picard and E. E. Epel, et al. "Depression, Telomeres and Mitochondrial DNA: Between-and within-Person associations from a 10-Year Longitudinal Study." Mol Psychiatry 234 (2018): 850-7.
- Levy, Richard J. and Clifford S. Deutschman. "Deficient Mitochondrial Biogenesis in Critical Illness: Cause, Effect, or Epiphenomenon?" Crit Care 11 (2007): 1-2.’
- Kadhum, Aamal Muhsen, Bayader M. Abd Al-Kadim, Thanaa Chasib Kareem and Ali Ahmed Nayyef, et al. "Associations of Tumor Necrosis Factor-[alpha] G-308A (rs1800629) Gene Polymorphisms with Major Depressive Disorder." Neuro Quantology 19 (2021): 133-7.
- Kadhum, Aamal Muhsen, Eman Salih Abd, Ruaa Amer Taleb and Kareem Nasir Hussain, et al. "Genetic Polymorphisms of Mitochondrial Genes (ND1, ATP6c) in Depression Patients." Neuro Quantology 19 (2021): 128-33.
Citation: Ali, Muneam Hussein, Hajer Ali Enad, Abeer Ameen Baqer and Safa K. Hachim, et al. &ldquoThe Mitochondrial DNA D-Loop Variations in Depressed Patients.” Clin Schizophr Relat Psychoses DOI: 10.3371/CSRP.AMHE.091221.
Copyright: © 2021 Hameed NM, et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.