Research Article - Clinical Schizophrenia & Related Psychoses ( 2022) Volume 0, Issue 0
The Dopamine Level and Dopaminergic Receptor Gene: DOR1 VNTR in Smokers
Huda Khalil Yaseen1, Mizher Khlif Hsony2, Omar Sinan Sadiq Al‐Zaidi3, Ali Abdulhussain Fadhil4, Zainab Sami Abbas5, Usama S. Altimari6* and Mona N. Al‐Terehi72Department of Medical Science, AL‐Manara College for Medical Sciences, Maysan, Iraq
3Department of Biology, Al‐Rasheed University College, Baghdad, Iraq
4Department of Medical Technology, Al‐Farahidi University, Baghdad, Iraq
5Department of Medical Laboratory Techniques, AL‐Mustaqbal University College, Babil, Iraq
6Department of Chemistry, Al‐Nisour University College, Baghdad, Iraq
7Department of Science, University of Babylon, Baghdad, Iraq
Usama S. Altimari, Department of Chemistry, Al‐Nisour University College, Baghdad, Iraq, Email: usamaaltimari@yahoo.com
Received: 11-Nov-2021, Manuscript No. CSRP-21-47225; Editor assigned: 15-Nov-2021, Pre QC No. CSRP-21-47225 (PQ); Reviewed: 29-Nov-2021, QC No. CSRP-21-47225; Revised: 07-Dec-2021, Manuscript No. CSRP-21-47225 (R); Published: 15-Dec-2021, DOI: 10.3371/CSRP.YKMH.121521
Abstract
The smoking is a common habit in the world which contributed in several diseases, the dopamine and its receptors were found to be related with addictive, in Iraq high percentage of population was smokers and others suffer from passive smokers, thus current study aims to detect, the Dopamine Level and Dopaminergic receptor Gene In young Smokers. The dopamine level and Dopaminergic Genes VNTR were detected using VNTR-PCR and ELIZA technique, the results show that non-significant differences in age and BMI (p=0.553, and p=0.638) and non-significant differences in dopamine levels between groups (p=0.823), three VNTR were observed 11(520 bp), 10 (483 bp) and 7(361 bp). The VNTR11 is more frequent in the smoker group (83.33%) in non-significant variation (p=0.966), also VNTR 10 are more frequent in the smoker group (16.66%) in non-significant variation (p=0.894), and VNTR 7 didn’t observe in the smoker group. Significant differences observed in non-smoker dopamine level according to VNTR frequency (p=0.025) while non-significant observed in the smoker group (p=0.324). The study concluded that the dopamine level didn’t effect by smoking and VNTR of DAT1 gene didn’t associate with the smoking habit also.
Keywords
Dopamine • Dopaminergic • Genes • VNTR • Smokers
Introduction
The smoking habit still prevalent in the world, it’s the risk factor of a large number of diseases, like cardiovascular disease and cancers that leads to largest morbidity and mortality [1-3]. The continuous smoking causes Nicotine dependence that known as a psychoactive substance disorder [4,5], this behavior is multifactorial represented by genetic and environmental interaction, complex associations have been found by several investigations [6,7]. The human behavior affected by different factors like neurotransmitter level, genes polymorphisms of its receptors and numerous hormones that lead to nicotine addiction [8]. The nicotine intake effects in different body organs like the central nervous system, studies found the metabolism of nicotine is regulated by some biological pathways, the Genetic researches introduced a method for developing insights into the genes involved in the metabolism pathways, however a strongest genetic contribution to smoking-related traits comes from diversity in the nicotinic receptor subunit genes and genes coding for enzymes involved in nicotine metabolism that proved by genome wide association and meta-analysis studies [9-11].
Dopamine is one of the neurotransmitter have a vital role in the human body, the level of dopamine is affected by different factors, there are several dopamine receptor genes, including DRD1-5 [12-17]. These receptors have been found to be associated with smoking behavior in different populations. All these receptors are G-protein-coupled receptors, In the present work the DRD1 is studied, its located at 5q35.2 4147 bases encoded to five subtypes D1-D5, that most abundant in the central nervous system the function trigger by adenylyl cyclase and cyclic AMP-dependent protein kinases activation [18]. Researchers found that DRD1 subtypes is linked with several behavioral disorders, despite of large number of human studies have focused indistinctively on the role of DA activity at the D2 and D3 receptor, different lines of evidence, mostly from preclinical work have shown that the D3 dopamine receptor may be particularly involved in nicotine addiction, D3 antagonism reduces the influence of cues on behavior using Pavlovian conditioning procedure, nicotine-induced place preferences , nicotine-induced reinstatement of nicotine seeking , and cue-induced reinstatement of nicotine seeking [19,20]. The polymorphism in this gene also mediated several disorders like depressive symptoms, alcohol dependence, bipolar disorder, and nicotine dependence [21-23]. Bipolar disorder formerly called depression, it is a mental health condition that causes utmost mood swings that include higher emotions (mania or hypomania) and lows (depression).When you become depressed, you may feel very low or hopeless and lose interest or pleasure in most of the activities. When your mood shifts to mania or hypomania (less extreme than mania), you may feel euphoric, full of energy or unusually irritable. These mood swings can affect sleep, energy, activity, judgment, behavior and the ability to think clearly. There are many types of bipolar and related disorders. Depression and mania or hypomania may be among them. These symptoms can lead to erratic changes in mood and behaviour, which can cause great distress and make life difficult.
Materials and Methods
The aim of this study is to determine the dopamine level and dopaminergic receptor gene DOR1 in smokers using VNTR-PCR and ELIZA techniques. The participants in the present study have age (22-34) years, data and blood samples were collected with participants approval and according to ethical approval of ministry of higher education and scientific research, its classified to smoker and non-smoker group, dopamine were detected by ELIZA and DNA isolated by favor gene extraction kit.
Amplification conditions and oligos
The VNT-PCR were detected using f-TGTGGTGTAGGGAACGGCCTGAG, r-CTTCCTGGAGGTCACGGCTCAAGG at the following PCR program 95ºC for 5 min, then 35 cycle included (95ºC for 30 sec. annealing tm 57ºC for 1 min and extension 72ºC for 30 sec) then final 72ºC for 7 min. the VNTR variations were detection by electrophoresis using 1% agaros, 70 V for 30 min with ethidum bromide staining and TBE 0.5X. The VNTR frequents detection as following VNTR 11 repeats (520 pb), VNTR 10 repeats (483 pb), VNTR 9 repeats (441 pb), VNTR 7 repeats (361 pb), VNTR 6 repeats (321 pb).
Exclusion criteria
Current study excluded alcohol abuse cases, neurotransmitter disorder cases, cancer cases, virus infection patients, diabetes mellitus type 1 and 2, hypertension cases and patients with kidney disease. All samples were male and apparently healthy.
Data analysis
Data were represented as mean ± stander error, statistical analysis implemented using independent t test, ANOVA one way at p value<0.05 and odd ratio at confidence intervals 95% using SPSS version 23.
Results
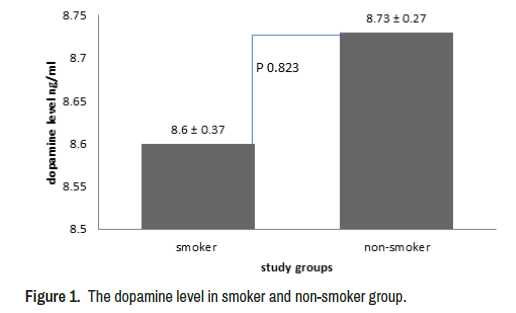
The study deal with smoker and non-smoker individuals that have nonsignificant differences in age and BMI (p=0.553, and p=0.638) respectively (Table 1). The dopamine level was estimated in both groups, output referred to non-significant differences between groups (p=0.823) (Figure 1).
| Categories | Smoker | Non-smoker | Sig |
|---|---|---|---|
| Age | 26.0000 ± 1.87972 | 27.2174 ± 0.91314 | 0.553 |
| BMI | 23.9500 ± 1.53683 | 24.7843 ± 0.80089 | 0.638 |
| Note: Independent T test at p value<0.05. | |||
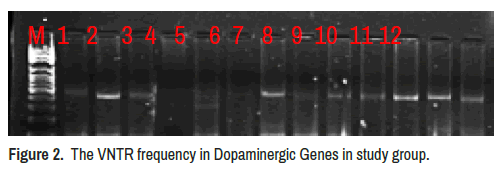
The VNTR in Dopaminergic Genes Dat1 was estimated using VNTR-PCR, three VNTR were observed 11(520 bp), 10 (483 bp) and 7(361 bp) (Figure 2). The VNTR11 is more frequent in the smoker group (83.33%) in non-significant variation (p=0.966), also VNTR10 more frequent in the smoker group (16.66%) in non-significant variation (p=0.894) and VNTR7 didn’t observed in in smoker group (Table 2).
|
VNTR frequency |
Smoker |
Non-smoker |
Odd ratio |
Sig |
|---|---|---|---|---|
|
11 (520 bp) |
83.33 |
79.16 |
0.9500 |
0.966 |
|
10 (483 bp) |
16.66 |
12.5 |
0.7778 |
0.894 |
|
7 (361 bp) |
0 |
4.16 |
Reference group |
|
|
Note: p value<0.05. |
||||
The effect of Dopaminergic Genes, Dat1 VNTR in dopamine level was evaluated, results show there was significant differences observed in non- smoker dopamine level according to VNTR frequency (p=0.025) while nonsignificant observed in the smoker group (p=0.324) (Table 3).
| VNTR frequency | Smoker | Non-smoker |
|---|---|---|
| 7 (361 bp) | 0 | 11.00 ± 0a |
| 10 (483 bp) | 8.00 ± 0 | 7.866 ± 0.56960b |
| 11 (520 bp) | 8.72 ± 0.43635 | 8.747 ± 0.29184ab |
| Sig | 0.324 | 0.025 |
|
Note: ANOVA one way, different letter refer to signiicant Differences among group at p value less than 0.05. a and b refer to significant differences 7 and 10 and ab referred to non-significant differences between 11 and 10. |
||
Discussion
Present study deal with effect of Smoking in the dopamine level and VNTR frequency in the DOR1 gene, this study was suggested because increased the smoking habit among young's and teenagers in the last years; the harmful effects of smoking have been fully described. Non-significant in dopamine level was observed among smoker and non-smoker, present finding deal with other study found that moderate smoking isn’t related with changes in the striatal dopamine synthesis capacity [24], while another found elevated in dopamine synthesis in smokers than non-smokers [25], on the other hand the dopamine synthesis is higher in female than male [26]. Belong to DOR1 VNTR frequency present output indicated that non-significant differences with a smoker, Ruzilawati, et al. found that the genotype of DOR1 (rs686) was significantly associated with smoking behavior [27]. Others pointed that the polymorphism in DRD1 is significantly correlated with nicotine dependence in an African American population, which eventually affects the expression of DOR1 [28]. The passive smoker Non- significant interaction between parental smoke exposure and DOR4 VNTR or OPRM1 A118G [29].
Furthermore the Dopaminergic changes are hypothesized to underlie addictive behavior [30]. Deal with this, nicotine trigger Nicotinic Acetyl Cholinergic (nACh) receptors leading to release dopamine [31]. The preclinical investigations found that the acute nicotine rewarding impacts are associated with two primary ways. First, nicotine stimulate VTA dopaminergic neurons directly, that expressed dopamine in the nucleus accumbens, Second, it stimulates nAChR receptors located on the dopaminergic terminals augmenting dopamine release, [32], the dopamine studies that included expression, transporter of dopamine and its receptor levels in smokers found robustness association results while others remains unclear, thus further investigations should be implemented.
The current study has several limitations, first of all the sample size was modest, because most of smokers in Iraq suffered from different disease and health disorder in addition to smoking complications. Till now there is a study that has examined the effect of tobacco smoking on DA release in D3-rich extra- striatal areas or has investigated whether activity at this receptor accounts for motivation to smoke, craving, or mood (anxiety).
Conclusion
The role of dopamine in addictive has been reported; despite of the high percent of smokers and passive smokers in the population, the present study concluded that the smoking didn’t effect in the dopamine level and dopamine recptor1 gene polymorphism. The effects of genetic variation on dopamine neurotransmission and that such an approach may be useful for understanding the inter-individual differences in the motor learning, plasticity, and the response to a dopaminergic drug. It also need more investigation to study other SNPs in Dopaminergic Genes that may be related to smoking habit which also included the passive smoker.
References
- Kilmer Greta, Roberts Henry, Hughes Elizabeth and Li Y, et al. “Surveillance of Certain Health Behaviors and Conditions Among States and Selected Local Areas-Behavioral Risk Factor Surveillance System (BRFSS), United States, 2006.” MMWR Surveill Summ 57 (2008) 188.
[Google scholar] [Pubmed]
- Hyland, A C Vena, J Bauer and Q Li. “Cigarette Smoking-Attributable Morbidity-United States, 2000.” MMWR Morb Mortal Wkly Rep 52 (2003): 842-844.
[Crossref] [Google scholar] [Pubmed]
- Ezzati, Majid, and Alan D Lopez. “Estimates of Global Mortality Attributable to Smoking in 2000.” Lancet 362 (2003): 847-852.
[Crossref] [Google scholar] [Pubmed]
- Moxham, John. “Nicotine addiction.” BMJ 320 (2000): 391-392.
- Leshner, Alan I. “Addiction is a Brain Disease, and it Matters.” Science 278 (1997): 45-47.
[Crossref] [Google scholar] [Pubmed]
- Hall, Wayne, Pamela Madden, and Michael Lynskey. “The Genetics of Tobacco Use: Methods, Findings and Policy Implications.” Tob Control 11 (2002): 119-124.
[Crossref] [Google scholar] [Pubmed]
- Yoshimasu, Kouichi and Chikako Kiyohara. “Genetic Influences on Smoking Behaviour and Nicotine Dependence: a Review.” J Epidemiol 13 (2003): 183-192.
[Crossref] [Google scholar] [Pubmed]
- Dfarhud, Dariush, Maryam Malmir and Mohammad Khanahmadi. “Happiness and Health: The Biological Factors-Systematic Review Article.” Iran J Public Health 43 (2014): 1468.
[Google scholar] [Pubmed]
- Loukola, Anu, Jenni Hällfors, Tellervo Korhonen, and Jaakko Kaprio. "Genetics and Smoking." Curr Addict Rep 1 (2014): 75-82.
[Crossref] [Google scholar] [Pubmed]
- Tobacco and Genetics Consortium. "Genome-Wide Meta-Analyses Identify Multiple Loci Associated with Smoking Behavior." Nat Genet 42 (2010): 441-447.
[Crossref] [Google scholar] [Pubmed]
- Liu, Jason Z., Federica Tozzi, Dawn M. Waterworth and Sreekumar G. Pillai, et al. "Meta-Analysis and Imputation Refines the Association of 15q25 with Smoking Quantity." Nat Genet 42 (2010): 436-440.
[Crossref] [Google scholar] [Pubmed]
- Lee, Wonho, Riju Ray, Andrew W Bergen and Gary E. Swan et al. “DRD1 Associations with Smoking Abstinence Across Slow and Normal Nicotine Metabolizers.” Pharmacogenet Genomics 22 (2012): 551.
[Crossref] [Google scholar] [Pubmed]
- Munafò, Marcus R, Nicholas J Timpson, Sean P David and Shah Ebrahim, et al. “Association of the DRD2 Gene Taq1A Polymorphism and Smoking Behaviour: A Meta-analysis and New Data.” Nicotine Tob Res 11 (2009): 64-76.
[Crossref] [Google scholar] [Pubmed]
- Vieira, Graziela, Juliana Cavalli, Elaine CD Gonçalves and Saulo FP Braga, et al. “Antidepressant-like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor.” Biomolecules 10 (2020): 792.
[Crossref] [Google scholar] [Pubmed]
- Vandenbergh, David J, Richard J O’Connor, Michael D Grant and Akilah L Jefferson, et al. "GENETIC STUDY: Dopamine receptor genes (DRD2, DRD3 and DRD4) and Gene-Gene Interactions Associated with Smoking‐Related Behaviours.” Addi Bio 12 (2007): 106-116.
[Crossref] [Google scholar] [Pubmed]
- Bono, Federica, Veronica Mutti, Chiara Fiorentini and Cristina Missale. “Dopamine D3 Receptor Heteromerization: Implications for Neuroplasticity and Neuroprotection.” Biomolecules 10 (2020): 1016.
[Crossref] [Google scholar] [Pubmed]
- Sullivan, Patrick F, Michael C Neale, Michael A Silverman and Carol Harris‐Kerr, et al. “An Association Study of DRD5 with Smoking Initiation and Progression to Nicotine Dependence.” Am J Med Genet 105 (2001): 259-265.
[Crossref] [Google scholar] [Pubmed]
- Plavén-Sigray, Pontus, Granville James Matheson and Petter Gustavsson, et al. “Is Dopamine D1 Receptor Availability Related to Social Behaviour? A Positron Emission Tomography Replication Study.” PloS One 13 (2018): e0193770.
[Crossref] [Google scholar] [Pubmed]
- Lim, Sewon, Juwon Ha, Sam-Wook Choi and Seung-Gul Kang, et al. “Association Study on Pathological Gambling and Polymorphisms of Dopamine D1, D2, D3, and D4 Receptor Genes in a Korean Population.” J Gambl Stud 28 (2012): 481-491.
[Crossref] [Google scholar] [Pubmed]
- Abi-Dargham, Anissa, Janine Rodenhiser, David Printz and Yolanda Zea-Ponce, et al. “Increased Baseline Occupancy of D2 Receptors by Dopamine in Schizophrenia.” Proc Natl Acad Sci 97 (2000): 8104-8109.
[Crossref] [Google scholar] [Pubmed]
- Jiménez, Karen M, Angela J Pereira-Morales and Diego A Forero. “A Functional Polymorphism in the DRD1 Gene, that Modulates its Regulation by miR-504, is Associated with Depressive Symptoms.” Psychiatry Investig 15 (2018): 402
[Crossref] [Google scholar] [Pubmed]
- Huang, Weihua, Jennie Z Ma, Thomas J Payne and Joke Beuten, et al. “Significant Association of DRD1 with Nicotine Dependence” Hum Genet 123 (2008): 133-140.
[Crossref] [Google scholar] [Pubmed]
- Yasseen, Baseer, James L Kennedy, Laurie A Zawertailo and Usoa E Busto. “Comorbidity between Bipolar Disorder and Alcohol use Disorder: Association of Dopamine and Serotonin Gene Polymorphisms.” Psychiatry Res 176 (2010): 30-33.
[Crossref] [Google scholar] [Pubmed]
- Bloomfield, Michael AP, Fiona Pepper, Alice Egerton and Arsime Demjaha, et al. “Dopamine Function in Cigarette Smokers: An [18F]-DOPA PET Study.” Neuropsychopharmacolo 39 (2014): 2397-2404.
- Salokangas, Raimo KR, Harry Vilkman, Tuula Ilonen and Tero Taiminen, et al. “High Levels of Dopamine Activity in the Basal Ganglia of Cigarette Smokers.” Am J Psychiatry 157 (2000): 632-634.
[Crossref] [Google scholar] [Pubmed]
- Laakso, Aki, Harry Vilkman, J Örgen Bergman and Merja Haaparanta, et al. “Sex Differences in Striatal Presynaptic Dopamine Synthesis Capacity in Healthy Subjects.” Biol Psychiatry 52 (2002): 759-763.
[Crossref] [Google scholar] [Pubmed]
- Ruzilawati, Abu Bakar, Md Asiful Islam and Siti Khariem Sophia Muhamed, et al. “Smoking Genes: A Case–Control Study of Dopamine Transporter Gene (SLC6A3) and Dopamine Receptor Genes (DRD1, DRD2 and DRD3) Polymorphisms and Smoking Behaviour in a Malay Male Cohort.” Biomolecules 10 (2020): 1633.
[Crossref] [Google scholar] [Pubmed]
- Huang, Weihua, Jennie Z Ma, Thomas J Payne and Joke Beuten, et al. “Significant Association of DRD1 with Nicotine Dependence.” Hum Genet 123 (2008): 133-140.
[Crossref] [Google scholar] [Pubmed]
- Kleinjan, Marloes, Rutger CME Engels and Joseph R DiFranza. “Parental Smoke Exposure and the Development of Nicotine Craving in Adolescent Novice Smokers: The Roles of DRD2, DRD4, and OPRM1 Genotypes.” BMC Pulm Med 15 (2015): 1-12.
[Crossref] [Google scholar] [Pubmed]
- Di Chiara, Gaetano and Valentina Bassareo. "Reward System and Addiction: What Dopamine does and doesn’t do." Curr Opin Pharmacol 7 (2007): 69-76.
[Crossref] [Google scholar] [Pubmed]
- Keiflin, Ronald and Patricia H. Janak. "Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry." Neuron 88 (2015): 247-263.
[Crossref] [Google scholar] [Pubmed]
- Zhang, Lifen, William M. Doyon, Jeremy J. Clark and Paul EM Phillips, et al. "Controls of Tonic and Phasic Dopamine Transmission in the Dorsal and Ventral Striatum." Mol Pharmacol 76 (2009): 396-404.
[Crossref] [Google scholar] [Pubmed]
Citation: Yaseen, Huda Khalil, Mizher Khlif Hsony, Omar Sinan Sadiq Al-Zaidi and Ali Abdulhussain Fadhil, et al. “The Dopamine Level and Dopaminergic Receptor Gene: DOR1 VNTR in Smokers.” Clin Schizophr Relat Psychoses 16S (2022). Doi: 10.3371/CSRP.YKMH.121521.
Copyright: © 2022 Yaseen HK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.