Research Article - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 5
Serum Cortisol and Folate Levels in Patients with Schizophrenia: Case Control Study in North of Iran
Najmeh Shahini*, Mostafa Zare, Mahsa Omiddezyani and Abdurrahman CharkaziNajmeh Shahini, Department of Psychiatry, Golestan Research Center of Psychiatry, Golestan University of Medical Sciences, Iran, Email: mgirmamulu12@gmail.com
Received: 15-Sep-2022, Manuscript No. CSRP-22-74797; Editor assigned: 19-Sep-2022, Pre QC No. CSRP-22-74797 (PQ); Reviewed: 04-Oct-2022, QC No. CSRP-22-74797; Revised: 02-Jan-2023, Manuscript No. CSRP-22-74797 (R); Published: 13-Jan-2023
Abstract
Introduction: Schizophrenia is a highly devastating condition characterized by frequent recurrences, cognitive decline, and emotional and functional disabilities. This study aimed to evaluate the serum levels of cortisol and folate in patients with schizophrenia and its comparison with healthy individuals.
Materials and methods: This is a case control study was performed on 66 individuals. Participants were divided into two groups schizophrenia (n=33) and control (n=33). The demographic information checklist, Simpson-Angus extrapyramidal Side Effect Scale (SAS), and Positive and Negative Syndrome Scale (PANSS) were used to collect demographic data, the severity of schizophrenia symptoms, and extrapyramidal symptoms. Then, blood samples were taken from each patient to measure serum levels of cortisol and folate.
Results: The level of folate in the schizophrenia group was significantly lower than in the healthy group and level of cortisol in the schizophrenia group was significantly higher than in the healthy group (P<0.0001). No significant relationship was observed between both cortisol levels and folate levels and the mean total score of the SAS questionnaire as well as the mean total score of the PANSS questionnaire, positive symptom scores, negative symptoms scores, and general psychopathologic symptoms scores in the schizophrenic group and the control group. The cortisol levels in those with a history of psychiatric illness were significantly lower than in the healthy group (P=0.017). The mean serum folate levels in patients with a history of hypothyroidism were significantly lower than in the healthy group (P=0.020). Folate levels were significantly higher in smokers (P=0.036).
Conclusion: The result showed that the serum folate level was significantly lower in the schizophrenia group and the serum cortisol level was significantly higher in schizophrenic patients. PANSS and SAS scores showed no association with serum cortisol and folate levels.
Keywords
Schizophrenia • Cortisol • Folate • Simpson-Angus • Extrapyramidal side effect scale • Positive and negative syndrome scale
Introduction
Association between serum cortisol and folate levels has been demonstrated in patients with schizophrenia. About 1% of people worldwide suffer from schizophrenia. It is a debilitating and costly disease since treatment resistant symptoms are very usual. Schizophrenia is a serious psychiatric disorder whose cause is still unknown. Evidence from animal experiments, clinical treatment courses, and neuroimaging studies suggests that abnormal neural development and gray matter deficits in different areas of the brain are involved in the pathogenesis of schizophrenia [1,2]. Clinical data shows a weakened biological response to stress in schizophrenic patients, which is related to dysregulation and dysfunction of the Hypothalamic Pituitary Adrenal (HPA) axis and changes in cortical stress response molecules. Impaired HPA axis in patients with schizophrenia has been proposed to be related to the enzyme as well as neurotransmitter systems abnormalities, which are responsible for the HPA axis, and limbic system structural abnormalities [3,4]. It was shown that increased levels of glucocorticoids and cortisol result in neurotoxicity, inhibition of neurogenesis, atrophy, and neuronal death. Increased corticosteroids including cortisol are related to structural changes, mostly reduction in the hippocampus volume and prefrontal cortex. The hippocampus and prefrontal cortex are the main targets for glucocorticoids and cortisol, and molecular abnormalities in cortisol responses have been reported in schizophrenia. Chronically increased levels of cortisol can decrease neurogenesis and synaptic plasticity in the hippocampus, resulting in cognitive impairment [5,6]. A meta-analysis study revealed reduced volume as well as neuron numbers in several subfields of the left hippocampus in schizophrenic patients. Circulating cortisol has been linked to reductions in hippocampal volume in first episode psychosis, which shows the deleterious impacts of stress early in the disease. HPA axis abnormalities may lead to cortisol level elevation. Increased serum baseline cortisol levels have been reported in schizophrenic patients, however, there are other studies with opposing results [7,8].
Folate or folic acid is one of the B vitamins which are essential for cell division and maintenance. Folate facilitates the Sadenosylmethionine production, a methyl group donor for various reactions of methylation, through the conversion of homocysteine into methionine. It was shown that folate is crucial for neuronal function [9,10]. Folate insufficiencies may be associated with the increased risk of neurodevelopmental complaints, psychiatric illnesses, and dementia. Reduced levels of folate may be related to schizophrenia by acting via increasing homocysteine or by homocysteine independent impacts on neuronal progenitor division or through altered one carbon metabolism. Folate is a substrate for Methylenetetrahydrofolate Reductase (MTHFR), an enzyme that catalyzes the conversion of 5, 10 methyle netetrahydrofolate to 5 methyltetrahydrofolate, which remethylated homocysteine to methionine. Therefore, the lack of folate may increase the level of homocysteine, which was shown to be associated with schizophrenia in its elevated levels [11,12]. Folate deficiency has been associated with several neuropsychiatric disorders, including schizophrenia. Evidence suggests that folate supplementation may be promising for the treatment of negative symptoms in schizophrenia while it does not appear to affect positive symptoms. On the other hand, in some studies, blood folate levels appear to decrease in patients with schizophrenia [13,14]. Because the prevalence of folate deficiency and hyperhomocysteinemia in the United States has decreased significantly since the mandatory enrichment of cereal grains with folate in 1998 and has subsequently decreased the prevalence of schizophrenia, folate deficiency has been identified as a risk factor for schizophrenia [15,16]. A review of the literature showed that such studies have been done very little in our country and the results in the world are contradictory. Therefore, we performed a study to compare the serum levels of cortisol and folate in patients with schizophrenia and healthy individuals [17].
Material and Methods
Study design and setting
The study was designed and conducted under the case control method. This study was performed on patients referred to specialized psychiatric clinics of 5th Azar Educational and Medical Center in Gorgan, North of Iran, from September 2021 to March 2022 [18].
Participants
Patients with schizophrenia were selected after approval by a psychiatrist based on inclusion and exclusion criteria and the Diagnostic and Statistical manual of mental disorders, fifth edition (DSM-5) criteria. The inclusion criteria were including, having at least 18 years of age, confirmation of schizophrenia by a psychiatrist in the schizophrenia group based on DSM-5 criteria, absence of any psychiatric symptoms [19]. Such as depression or a history of psychiatric illness in the patient or parents in the control group, obtaining informed written consent from the patient or patient caregiver, and absence of other physical and psychiatric illnesses in patients diagnosed with schizophrenia. Patients who were not referred for cortisol and folate tests and patients who were dissatisfied with participation during the study, and those use folate supplements were excluded from the study. The control group was selected from patients referred to the internal clinic of 5th Azar Hospital in Gorgan who did not have a psychiatric illness. The subjects in the control group were matched with the schizophrenia group in terms of gender and age [20].
Study size
The sample size in this study was calculated. The sampling method in this study was the convenience sampling method. Using the following formula, the sample size was defined as 66 people, of which 33 were in the case group and 33 were in the control group [21,22].
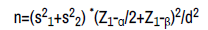
Were s2 1 and s2 2 are the variance of cortisol or folate levels in patients. α is Significance level and 1-β is study power. d is the minimum difference in folate/cortisol level between the patient and control groups.
Measurement
Demographic information questionnaire: A questionnaire was used to record the demographic characteristics of the patients.
Schizophrenic symptoms questionnaire
In this study, the Positive and Negative Syndrome Scale (PANSS) was used to evaluate the severity of positive and negative symptoms in schizophrenic patients. For extrapyramidal symptoms of schizophrenia Simpson-Angus Extrapyramidal Side Effects Scale (SAS) was used [23,24].
• PANSS: This questionnaire was used to assess the severity of schizophrenia symptoms in patients, which included three question categories, 7 questions for positive symptoms, 7 questions for negative symptoms, and 16 questions for general symptoms [25]. The questions were answered based on 7-point scale (1=absent, 2=minimal, 3=mild, 4=moderate, 5=moderate severe, 6=severe, and 7=extreme). The validity of this questionnaire in Iran was determined. And the Cronbach's alpha was defined as 0.77 [26].
• SAS: This questionnaire was used to assess the severity of extrapyramidal symptoms of schizophrenia in patients, which included 10 questions and symptoms. The questions of this questionnaire were scored based on a 5-point scale with a score of 0 to 4 [27]. This questionnaire is validated in Iran.
Cortisol and folate measurements
Between 7 am to 8 am, 3 cc of venous blood was collected from each patient. The sample was stored in a tube containing 3.5% sodium citrate to test serum cortisol and folic acid levels [28]. The samples were stored at -20°C. Measurement of cortisol is done by Cortisol AccuBind ELISA Kit (Monobind Inc., USA) and measurement of folate is done by Folate AccuBind ELISA Kits (Monobind Inc., USA) via ELISA technique. 25 μl of standards, controls, and samples were added into the wells and then 50 μl of the conjugate solution was added to all wells [29]. The plate was shaken gently for 15 seconds and incubated for 60 minutes at 37°C. After removing the plate from the incubator, 50 microliters of biotin solution were added to each well and incubated for 30 minutes at 37°C. Then the contents of the wells were emptied and then washed with 350 microliters of buffer and this repeated for 4 more times [30]. 100 μl of ready to use dye substrate was added to all wells and incubated for 15 minutes at room temperature and in a dark place, then 50 μl of the reaction stop solution was added in the same manner as the dye substrate solution. Then the absorption of each well was read at a wavelength of 450 nm after a maximum of 10 minutes with the multiple plate microplate reader URIT-660 (URIT Medical Electronic Co., China) devices [31].
Statistical analysis
Data are described by central and dispersion indices (mean, median, standard deviation), and tables and graphs. In the case of normality, the independent T-Test was used and in the absence of normality assumptions, the Mann-Whitney U test was used [32]. A correlation test was used to examine the relationship between variables and cortisol and folic acid levels in each group of patients and control. Linear regression analysis was used to compare the levels of cortisol and folic acid in the two groups of patients and controls, taking into account the age and Gender of the subjects in the study [33]. The analyzes were performed using SPSS software version 16 and at a significant level of 0.05.
Results and Discussion
Participants
Sixty-six individuals participated in this study. Participants in the study were admitted into two groups control (n=33) and schizophrenia (n=33).
Descriptive data
The mean and standard deviation of the age of the participants in the study was 39.89 ± 12.22 years with a range of 18-75 years. The mean and standard deviation of the Body Mass Index (BMI) of the participants was 24.86 ± 3.69. Among participants, 51.5% were male, 62.1% were single, 81.3% had a diploma and under diploma level of education, 77.3% were Fars, 28.8% had medical illness history, and 45.5% had hypothyroidism [34]. Also, 28.8% had a history of Electroconvulsive Therapy (ECT). A small number had a history of smoking (6.1%) In this study, the serum levels of cortisol and folate in patients with schizophrenia referred to the psychiatric clinic were studied [35]. The level of cortisol in the schizophrenia group was significantly higher than in the healthy group. The level of folate in patients with schizophrenia was significantly lower than in healthy individuals. The cortisol levels in those with a history of psychiatric illness were significantly lower than in the healthy group. The mean serum folate levels in patients with a history of hypothyroidism were significantly lower and in smokers were significantly higher [36]. Moreover, no significant relationship was observed between both cortisol levels and folate levels and the mean total score of the SAS questionnaire as well as the mean total score of the PANSS questionnaire, positive symptom scores, negative symptoms scores, and general psychopathologic symptoms scores in the schizophrenic group and the control group [37].
In the present study, the levels of serum folate were significantly lower in the schizophrenia group. The result of in Korea also showed that patients with schizophrenia had significantly lower levels of folate in comparison to the control group. They showed that the low folate level patient group had significantly higher homocysteine levels, which itself via neurotoxic mechanism has an effect on schizophrenia. In another study in Iran, it was found that patients with schizophrenia had significantly lower folate levels than healthy individuals. Study showed significantly lower serum levels of folate in the schizophrenia group [38]. They showed that drug naïve, first episode schizophrenia presents diminished serum levels of folate, decreased brain derived neurotropic factor, and increased homocysteine, which might play a significant role in the neurodevelopmental process of schizophrenia as well as its clinical manifestation [39]. In a meta-analysis, the levels of serum folate were significantly lower in schizophrenia patients than in controls and the reduced folate level was observed in both Asian and European patients. They suggested decreased folate as a risk factor for schizophrenia. In a meta analysis, it was reported that decreased levels of folate were associated with schizophrenia risk in total studies as well as in subgroups of Caucasians, English publications, Asians, acute schizophrenia patients, and age less than 50 with the great enough powers [40]. Another meta-analysis on 20 studies also confirmed that serum folate level was significantly lower in cases with schizophrenia than in healthy individuals. Assessed the relationship between serum folate levels in patients with schizophrenia based on sex and reported that serum folate levels might be related to schizophrenia irrespective of sex and folate administration could be advantageous for schizophrenia treatment. Study in the schizophrenia group, a significant negative relationship was found between serum levels of folate and PANSS total scores and PANSS negative symptom scores. However, in the current study, no relationship was found between folate level and PANSS negative, positive or total score [41].
In the current study, the level of serum cortisol was significantly higher in the schizophrenia group. Evaluated the serum cortisol levels in patients with schizophrenia. In this study, 60 patients with schizophrenia, 70 healthy relatives, and 60 healthy volunteers were studied. In this study, serum cortisol levels in the schizophrenia group compared with healthy relatives and control group were significantly higher. Increasing serum cortisol levels in schizophrenic patients may be related to the role of cortisol in schizophrenic pathophysiology. Also, increasing serum cortisol levels in first degree relatives compared with the control group shows that similar pathophysiological trends may have a role in people without any symptoms but with a genetic talent for schizophrenia. Also demonstrated that in schizophrenia patients without metabolic syndrome, the cortisol blood level was significantly higher than the healthy controls 40 compared the serum cortisol in schizophrenic patients and the control group. In this study, 66 patients with chronic schizophrenia and 28 healthy men participated. Serum cortisol levels in patients were 12.48 ± 3.2 μg/dl and in the control group was 10.31 ± 3.1 μg/dl which was significantly higher in schizophrenic patients than in healthy subjects, no significant difference was observed between the schizophrenia and control groups regarding serum cortisol level. Study showed that schizophrenia patients who underwent treatment and does not show important clinical symptoms of schizophrenia may have mildly lowered salivary cortisol levels. Study results, serum cortisol level were significantly lower in schizophrenia after treatment with antipsychotics than its baseline value. Study at baseline, no association was observed between serum cortisol levels in schizophrenia patients in PANSS positive and negative sub scores. However, after treatment with antipsychotic serum cortisol level presented a strong association PANSS positive and negative sub scores. In the present study, no association was observed between serum cortisol levels and PANSS scores (Table 1).
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 24 (51.5) |
| Female | 32 (48.5) |
| Marital status | |
| Single | 41 (62.1) |
| Married | 25 (37.9) |
| Education level | |
| Under diploma | 28 (42.4) |
| Diploma | 25 (37.9) |
| University education | 13 (19.7) |
| Ethnicity | |
| Turkmen | 4 (6.1) |
| Fars | 51 (77.3) |
| Sistani | 7 (10.6) |
| Other | 4 (6.1) |
| History of medical illness | |
| Yes | 19 (28.8) |
| No | 47 (71.2) |
| History of hypothyroidism | |
| Yes | 30 (45.5) |
| No | 36 (54.5) |
| History of ECT | |
| Yes | 19 (28.8) |
| No | 47 (71.2) |
| History of smoking | |
| Yes | 4 (6.1) |
| No | 62 (93.9) |
Table 1. Frequency distribution of demographic characteristics of participants in the study.
The results of SAS and PANSS questionnaires
SAS questionnaire: The mean score of the SAS questionnaire in patients was 2.98 ± 2.12. The minimum score received from this questionnaire in patients was 0 and the maximum was 8.
PANSS questionnaire
The mean score of the PANSS questionnaire in patients was 102.12 ± 42.73. The minimum score received from this questionnaire in patients was 33 and the maximum was 74.
• Positive symptoms: The mean score of schizophrenia patients was 30.51 ± 2.24 for positive symptom questions. The minimum score received from this set of questions was 20 and the maximum was 38.
• Negative symptoms: The mean score of schizophrenia patients was 22.03 ± 5.20 for negative symptom questions. The minimum score received from this set of questions was 15 and the maximum was 39.
• A positive and significant correlation was observed between the scores of positive and negative symptoms in patients with schizophrenia (r=0.613, P<0.01).
• General psychopathology: The mean score of schizophrenia patients was 49.87 ± 7.07 for psychopathologic symptom questions. The minimum score received from this set of questions was 31 and the maximum was 69 (Table 2).
| Questionnaire variables | Mean ± SD | Range |
|---|---|---|
| SAS | 2.93 ± 2.12 | 0-8 |
| PANSS | ||
| Positive symptoms | 30.51 ± 2.24 | 20-38 |
| Negative symptoms | 22.03 ± 5.20 | 15-39 |
| General psychopathology | 49.87 ± 7.07 | 31-69 |
| Total | 102.12 ± 42.73 | 74-133 |
Table 2. The mean and standard deviations of PANSS and SAS.
Relationship between the findings of the questionnaires
As can be seen in Table 3, a direct relationship was observed between general psychopathological symptoms or negative symptoms and total score. A positive and significant relationship was observed between negative symptoms and total score and between positive symptoms and total score of PANSS (P<0.05). On the other hand, no significant relationship was observed between the parameters of PANSS and SAS questionnaires (P>0.05).
| Variable | PANSS | SAS | ||||||
|---|---|---|---|---|---|---|---|---|
| PANSS | Negative symptoms | Positive symptoms | Total Score | Total Score | ||||
| Correlation coefficient | P | Correlation coefficient | P | P | Correlation coefficient | P | ||
| General psychopathology | 0.678 | 0 | 0.191 | 0.288 | 0.897 | 0 | 0.193 | 0.281 |
| Negative symptoms | 0.125 | 0.488 | 0.827 | 0 | 0.196 | 0.275 | ||
| Positive symptoms | 0.49 | 0.004 | 0.167 | 0.345 | ||||
| Total score | 0.243 | 0.173 | ||||||
Table 3. Relationship between questionnaire findings in the schizophrenia group.
Serum levels of cortisol and folate
The level of cortisol in the schizophrenia group was significantly higher than in the healthy group (P<0.0001). Also, the level of folic acid in patients with schizophrenia was significantly lower than in healthy individuals (P<0.0001) (Table 4).
| Variable | Schizophrenia patients | Healthy individuals | P-value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Serum Cortisol levels | 54.98 ± 26.90 | 18.84 ± 9.16 | <0.0001 |
| Serum folate levels | 2.21 ± 2.20 | 14.69 ± 11.68 | <0.0001 |
Table 4. Mean serum levels of Cortisol and folate in the patients with schizophrenia and healthy individuals.
Correlation between serum cortisol levels and questionnaire parameters
Relationship between serum cortisol levels and PANSS questionnaire: In this study, no significant relationship was observed between cortisol levels and the mean total score of the PANSS questionnaire, positive symptom scores, negative symptoms scores, and general psychopathologic symptoms scores in the schizophrenic group and the control group.
Relationship between serum cortisol levels and SAS questionnaire: No significant relationship was observed between cortisol levels and the mean total score of the SAS questionnaire.
Correlation between serum folate level and questionnaire parameters
Relationship between serum folate levels and PANSS questionnaire: In this study, no significant relationship was observed between folate levels and the mean total score of the PANSS questionnaire, positive symptom scores, negative symptoms scores, and general psychopathologic symptoms scores in the schizophrenic group and the control group.
Relationship between serum folate levels and SAS questionnaire: No significant relationship was observed between folate levels and the mean total score of the SAS questionnaire (Table 5).
| Questionnaire variable | Serum cortisol levels | Serum folate levels | |||
|---|---|---|---|---|---|
| Correlation coefficient | P value | Correlation coefficient | P-value | ||
| SAS | Total | 0.12 | 0.506 | 0.253 | 0.156 |
| PANSS | Positive symptoms | 0.102 | 0.571 | 0.072 | 0.691 |
| Negative symptoms | 0.243 | 0.172 | -0.228 | 0.202 | |
| General psychopathologic | 0.141 | 0.433 | -0.111 | 0.537 | |
| Symptoms | |||||
| Total | 0.212 | 0.236 | -0.131 | 0.467 | |
Table 5. Correlation between serum cortisol and Serum folate levels and questionnaire parameters.
Relationship between demographic variables and cortisol and folate levels
The results of the Mann-Whitney test showed that the mean serum folate levels in patients with a history of hypothyroidism were significantly lower than in the healthy group (P=0.020). Also, cortisol levels in those with a history of psychiatric illness were significantly lower than in the healthy group (P=0.017). Folate levels were significantly higher in smokers (P=0.036) (Table 6).
| Variable | Yes | No | Z | P-value | ||
|---|---|---|---|---|---|---|
| Cortisol levels | ||||||
| History of psychiatric illness | 12.16 ± 10.27 | 57.77 ± 26.45 | -2.38 | 0.017 | ||
| History of using psychiatric drugs | 27.12 ± 10.16 | 77.45 ± 57.26 | -2.38 | 0.017 | ||
| Folate levels | ||||||
| History of hypothyroidism | 1.94 ± 1.89 | 5.30 ± 2.78 | -2.318 | 0.02 | ||
| History of smoking | 4.70 ± 2.73 | 1.94 ± 1.86 | -2.097 | 0.036 | ||
Table 6. Relationship between demographic variables and cortisol and folic acid levels.
The results of logistic regression analysis for the relationship between measured serum cortisol and folate levels and schizophrenia showed that there was no significant relationship, one of the reasons could be the small sample size.
Conclusion
According to the result of the present study, the serum cortisol was significantly higher and folate levels was significantly lower in the schizophrenia group. It is suggested that in future studies the effect of supplements on schizophrenia be investigated and that clinical trials be performed on this issue.
Limitations
Due to the COVID-19 pandemic and health protocols, there was a decrease in the number of referrals to health centers and as a result, the lack of patients referred to psychiatric clinics and reduced hospitalization of psychiatric patients in the ward led to a prolongation of the sampling process. The sample size of this study was small and it is recommended to perform the future studies with larger sample size.
Ethical Consideration
This study is approved by the ethics committee of Golestan University of Medical Sciences.
Funding
There is no funding for the present study.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Ding, Yujie, Mingliang Ju, Lin He, and Wenzhong Chen. "Association of folate level in blood with the risk of schizophrenia." Comb Chem High Thro Screen 20 (2017): 116-122. [Crossref] [Google Scholar] [PubMed]
- Babinkostova, Zoja, Branislav Stefanovski, Danijela Janicevic-Ivanovska, and Valentina Samardziska. et al. "Association between serum cortisol and DHEA-s levels and response to antipsychotic treatment in schizophrenia." Open Access Maced J Med Sci 3 (2015): 123-124. [Crossref] [Google Scholar] [PubMed]
- Hori, Hiroaki, Toshiya Teraishi, Daimei Sasayama, and Kotaro Hattori et al. "Elevated cortisol level and cortisol/DHEAS ratio in schizophrenia as revealed by low-dose dexamethasone suppression test." Neuropsychopharmacology 5 (2012): [Crossref] [Google Scholar]
- Yung, Alison R, and Patrick D McGorry. "The prodromal phase of first-episode psychosis: past and current conceptualizations." Schizophr Bull 22 (1996): 353-370. [Crossref] [Google Scholar] [PubMed]
- Honea, Robyn, Tim J Crow, Dick Passingham, and Clare E. et al. Mackay. "Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies." Am J Psychiatry 162 (2005): 2233-2245. [Crossref] [Google Scholar] [PubMed]
- Selemon, LD, and N Zecevic. "Schizophrenia: a tale of two critical periods for prefrontal cortical development." Transl Psychiatry 5 (2015): 623-623. [Crossref] [Google Scholar] [PubMed]
- Walker, Elaine F, and Donald Diforio. "Schizophrenia: a neural diathesis-stress model." Psycholo Rev 104 (1997): 666-667. [Crossref] [Google Scholar] [PubMed]
- Ciufolini, Simone, Paola Dazzan, Matthew J Kempton, and Carmine Pariante. et al. "HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies." Neurosci Biobehav Rev. 47 (2014): 359-368. [Crossref] [Google Scholar] [PubMed]
- Sinclair, Duncan, Shan Yuan Tsai, Heng Giap Woon, and Cynthia Shannon Weickert. et al. "Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness." Neuropsychopharmacology 36 (2011): 2698-2709. [Crossref] [Google Scholar] [PubMed]
- Tandon, Rajiv, Cheryl Mazzara, John de Quardo, and Katherine A, et al. "Dexamethasone suppression test in schizophrenia: relationship to symptomatology, ventricular enlargement, and outcome." Bio Psychiatry 29 (1991): 953-964. [Crossref] [Google Scholar] [PubMed]
- Sapolsky, Robert M, Lewis C Krey and BRUCE S McEWEN. "Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging." J Neurosci 5 (1985): 1222-1227. [Crossref] [Google Scholar] [PubMed]
- Sapolsky, Robert M, Hideo Uno, Charles S Rebert, and Caleb E Finch. et al. "Hippocampal damage associated with prolonged glucocorticoid exposure in primates." J Neurosci 10 (1990): 2897-2902. [Crossref] [Google Scholar] [PubMed]
- Carrion, Victor G, Carl F Weems, Kit Richert and Bryce C Hoffman, et al. "Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth." Bio Psychiatry 68 (2010): 491-493. [Crossref] [Google Scholar] [PubMed]
- Kim, Eun Joo, and Blake Pellman Jeansok J Kim "Stress effects on the hippocampus: a critical review." Learn Mem 22 (2015): 411-416. [Crossref] [Google Scholar] [PubMed]
- Roeske, Maxwell J, Christine Konradi, Stephan Heckers, and Alan S Lewis. et al. "Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: a systematic review and meta-analysis of postmortem studies." Mol Psychiatry 26 (2021): 3524-3535. [Crossref] [Google Scholar] [PubMed]
- Mondelli, Valeria, Paola Dazzan, Nilay Hepgul, and Marta Di Forti, et al. "Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment." Schizophr Res 116 (2010): 234-242. [Crossref] [Google Scholar] [PubMed]
- Tobolska, Dominika, Krzysztof Maria Wilczynski, Miłosz Lorek and Elzbieta Mazgaj, et al. "Evaluation of the cortisol concentrations in patients with schizophrenia." Psychiatr Danub 28 (2016): 162-164. [Google Scholar] [PubMed]
- Kaneko, Motohisa, Fujio Yokoyama, Yoshihiko Hoshino, and Kenji Takahagi, et al. "Hypothalamic-pituitary-adrenal axis function in chronic schizophrenia: association with clinical features." Neuropsychobiology 25 (1992): 1-7. [Crossref] [Google Scholar] [PubMed]
- Ritsner, Michael, Rachel Maayan, Anatoly Gibel, and Rael D. et al. "Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients." Eur Neuropsychopharmacol 14 (2004): 267-273. [Crossref] [Google Scholar] [PubMed]
- Mitchell, E Siobhan, Nelly Conus and Jim Kaput. "B vitamin polymorphisms and behavior: Evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline." Neurosci Biobehav Rev 47 (2014): 307-320. [Crossref] [Google Scholar] [PubMed]
- Stabler, Sally P, Paul D Marcell, Elaine R Podell, and RH Allen, et al. "Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry." J Clin Investig 81 (1988): 466-474. [Crossref] [Google Scholar] [PubMed]
- Czeizel, Andrew E, Istvan Dudas, Attila Vereczkey, and Ferenc Banhidy. et al. "Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects." Nutrients 5 (2013): 4760-4775. [Crossref] [Google Scholar] [PubMed]
- Krebs, MO, Alfredo Bellon, Gaell Mainguy, and TM Jay, et al. "One-carbon metabolism and schizophrenia: current challenges and future directions." Trends Mol Med 15 (2009): 562-570. [Crossref] [Google Scholar] [PubMed]
- Lee, Young Sik, Doug Hyun Han, Chang Moo Jeon, and In Kyoon Lyoo, et al. "Serum homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients." Neuroreport 17 (2006): 743-746. [Crossref] [Google Scholar] [PubMed]
- Wang, Dan, Jun-Xia Zhai and Dian Wu Liu. "Serum folate levels in schizophrenia: A meta-analysis." Psychiatry Res 235 (2016): 83-89. [Crossref] [Google Scholar] [PubMed]
- Moustafa, Ahmed A, Doaa H Hewedi, Abeer M Eissa, and Dorota Frydecka, et al. "Homocysteine levels in schizophrenia and affective disorders focus on cognition." Front Behav Neurosci 8 (2014): 343. [Crossref] [Google Scholar]
- Wysokinski, Adam, and Iwona Kłoszewska. "Homocysteine levels in patients with schizophrenia on clozapine monotherapy." Neurochem Res 38 (2013): 2056-2062. [Crossref] [Google Scholar] [PubMed]
- Hill, Michele, Kelsey Shannahan, Sarah Jasinski, and Eric A. et al. "Folate supplementation in schizophrenia: a possible role for MTHFR genotype." Schizophr Res 127 (2011): 41-45. [Crossref] [Google Scholar] [PubMed]
- Roffman, Joshua L, J Steven Lamberti, Eric Achtyes, and Eric A Macklin. Et al. "Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia." JAMA Psychiatry 70 (2013): 481-489. [Crossref] [Google Scholar] [PubMed]
- Roffman, Joshua L, Liana J Petruzzi, Alexandra S Tanner, and Hannah E Brown. et al. "Biochemical, physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial." Mol Psychiatry 23 (2018): 316-322. [Crossref] [Google Scholar] [PubMed]
- Rader, Jeanne I. "Folic acid fortification, folate status and plasma homocysteine." J Nutr 132 (2002): 2466-2470. [Crossref] [Google Scholar] [PubMed]
- Yazici, Esra, Tugba Mutu Pek, Derya Guzel, and Ahmet Bulent Yazici, et al. "Klotho, vitamin D and homocysteine levels during acute episode and remission periods in schizophrenia patients." Ord J Psychiatry 73 (2019): 178-184. [Crossref] [Google Scholar] [PubMed]
- Fakhri, Ahmad, Sirous Pakseresht, Mohammad Reza Haghdoost, and Nasihat Hekmatkhah, et al. "Memantine enhances the effect of olanzapine in patients with schizophrenia: a randomized, placebo controlled study." Acta Med Iran (2016): 696-703. [Google Scholar] [PubMed]
- Kim, Tae Ho, Seok Woo Moon. "Serum homocysteine and folate levels in korean schizophrenic patients." Psychiatry Investig 8 (2011): 133-134. [Google Scholar]
- Song, Xueqin, Xiaoduo Fan, Xue Li, and David Kennedy, et al. "Serum levels of BDNF, folate and homocysteine: in relation to hippocampal volume and psychopathology in drug naive, first episode schizophrenia." Schizophr Res 159 (2014): 51-55. [Crossref] [Google Scholar] [PubMed]
- Tomioka, Yukiko, Makoto Kinoshita, Hidehiro Umehara, and Tomohiko Nakayama, et al. "Association between serum folate levels and schizophrenia based on sex." Psychiatry Clin Neurosci 74 (2020): 466-471. [Crossref] [Google Scholar] [PubMed]
- Yıldırım, Osman, Orhan Dogan, Murat Semiz, and Fatih Kilicli. et al. "Serum cortisol and dehydroepiandrosterone‐sulfate levels in schizophrenic patients and their first‐degree relatives." Psychiatry Clin Neurosci 65 (2011): 584-591. [Crossref] [Google Scholar] [PubMed]
- Boiko, Anastasiia S, Irina A Mednova, Elena G Kornetova, and Nikolay A Bokhan, et al. "Cortisol and DHEAS related to metabolic syndrome in patients with schizophrenia." Neuropsychiatr Dis Treat 16 (2020): 1051. [Crossref] [Google Scholar] [PubMed]
- Yılmaz, Necat, Hasan Herken, Hulya Kanbur Cicek, and Ahmet Celik, et al. "Increased levels of nitric oxide, cortisol and adrenomedullin in patients with chronic schizophrenia." Med Princ Pract 16 (2007): 137-141. [Crossref] [Google Scholar] [PubMed]
- Bulut, Suheyla D, Serdar Bulut, Ayse Gokcen Gundogmus, and Cigdem Aydemir, et al. "Serum DHEA-S, testosterone and cortisol levels in female patients with schizophrenia." Endocr Metab Immune Disorders Drug Targets 18 (2018): 348-354. [Crossref] [Google Scholar] [PubMed]
- Woldesenbet, Yohannes Markos, Arefayne Alenko, Iyasu Tadesse Bukata, and Lealem Gedefaw, et al. "The status of serum cortisol before and after treatment of schizophrenia and its correlation to disease severity and improvement: A longitudinal study." SAGE Open Med 9 (2021). [Crossref] [Google Scholar] [PubMed]
Citation: Omiddezyani, Mahsa, Mostafa Zare, Mahsa Omiddezyani and Abdurrahman Charkazi. "Serum Cortisol and Folate Levels in Patients with Schizophrenia: Case Control Study in North of Iran."Clin Schizophr Relat Psychoses 17 (2023).






