Review Article - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 1
Rituximab in Refractory Myasthenia Gravis: A Systematic Review
Lourdes de Fatima Ibanez Valdes, Sibi Sebastian Joseph and Humberto Foyaca Sibat*Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 04-Oct-2022, Manuscript No. CSRP-22-76538; Editor assigned: 17-Oct-2022, Pre QC No. CSRP-22-76538(PQ); Reviewed: 24-Oct-2022, QC No. CSRP-22-76538; Revised: 30-Dec-2022, Manuscript No. CSRP-22-76538(R); Published: 02-Jan-2023, DOI: 10.3371/CSRP.DLSS.011023
Abstract
Background: Despite Myasthenia Gravis (MG) is a chronic autoimmune disorder some spontaneous remissions can occur (4-17 years) even before introduction of immunosuppressant. For the past eighty years, remarkable progress in the therapy of MG has been, and currently this condition is one of the most treatable autoimmune disorders worldwide. However, an important number of cases remain refractory or present lack tolerance to steroids and other immune suppressants, in such situations monoclonal antibodies have been contributing to better outcome of that community. To determine the proportion of patients responding well to RTX or presenting adverse responses, complications, or fatal results, we performed a Systematic Review (SR) of the published articles on MG treated with RTX looking for safer and more effective treatment for RMG.
Material and methods: Following PRISMA guidelines, we searched EMBASE, Medline, Scopus online databases, WHO database, Google Scholar, Science Dirct, Scielo, LILACS, BIREME, Web on Science, and Cochrane library to identify articles evaluating rituximab therapy*, refractory MG*, a systematic review on MG*, rituximab in MC*, *from January 1, 2018, to July 30, 2021.
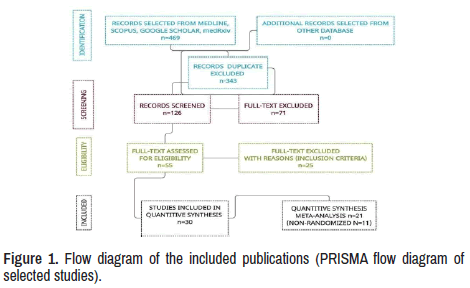
Results: We found 469 publications regarding to these issues. After removing duplicate articles, considering abstracts, titles, and screening full text, PCR positives, symptomatic patients, and manuscripts were written in other languages, only 30 matched all the selected parameters.
Comments and final remarks: Our study confirmed a remarkable clinical improvement of MG/RMG cases after initiating RTX therapy, a better Quality of Life (QOL), and beneficial outcomes in almost all cases reported in the medical literature. We also confirmed that RTX is well tolerated in AChR-MG and MuSK-MG patients. The cases presenting any adverse event after initiating RTX ranged from 26.4% and 42.8% and never were considered severe complications. Patients presenting AChR-RMG also improve when receiving repeated lower doses of RTX. However, multi-centre RCT using different doses of RTX in an extensive series should be performed to confirm its efficacy. The frequency of MGE/MC after initiating RTX therapy has not been determined. Incidence/prevalence of mortality has been no reported after a confident analysis and information from post-mortem examinations were not realized up to date. No evidence-based guidelines and relevant cost-effectiveness have been demonstrated. Apart from the clinical benefits obtained with RTX, a substantial number of patients were able to reduce doses and frequency of administration of associated immunomodulatory agents.
Keywords
Refractory myasthenia gravis • Myasthenic crisis • Rituximab (RTX) • Myasthenic exacerbation • Adverse effects to RTX
Abbreviations
AChR: Acetylcholine Receptor; ADL: Activities of Daily Living; AMSTAR 2: Measurement Tool to Assess systematic Reviews; CI: Confidence Interval; DAMP: Damage Associated Molecular Pattern; DC: Dendritic Cell; EOMG: Early Onset Myasthenia Gravis; EBV: Epstein Barr Virus; GC: Germinal Centre; IF: Interferon; IFNAR: Interferon-α/β Receptor; IgG: Immunoglobulin G; IL: Interleukin; JAK1: Janus Kinase 1; LOMG: Late Onset Myasthenia Gravis; LRP4: Low Density Lipoprotein Receptor-related Protein 4; MG: Myasthenia Gravis; MFA: Myasthenia Gravis Foundation of America; MGT: Thymoma Associated Myasthenia Gravis; MG-QOL: Myasthenia Gravis-specific Quality of Life; miRNA: MicroRNA; MuSK: Muscle Specific Kinase; PAMP: Pathogen Associated Molecular Pattern; QMG: Quantitative Myasthenia Gravis; PBMC: Peripheral Mononuclear Blood Cell; RCT: Randomized Controlled Trial; RIG-1: Retinoic Acid Inducible Gene I; SOCS: Suppressor of Cytokine Signalling; SD: Standard Deviation; STAT: Signal Transducer and Activator of Transcription; TEC: Thymic Epithelial Cell; Th17 cell: T Helper 17 cell; TLR: Toll-like Receptor; TNFα: Tumour Necrosing Factor Alpha; INFα: Interferon Gamma; TSA: Tissue-specific Antigen; USP18: Ubiquitin-specific Peptidase 18.
Introduction
Myasthenia Gravis (MG) is an uncommon B-cell-mediated autoimmune disorder affecting the neurotransmission of the Neuromuscular Junction (NMJ). Clinical features of MG are characterized by a variable combination of fluctuating muscle weakness of extraocular, bulbar, limbs and respiratory muscles associated with the remarkable presence of Autoantibodies Against Acetylcholine Receptors (AChRs), Lipoprotein Related Protein 4 (LPR4) or Muscle Specific Kinase (MuSK). Clinical prognosis moves from benign to fatal outcomes and can be usefully managed with anticholinesterase medications, fast immunomodulatory therapy, and chronic immunosuppressive drugs with or without thymus removal [1,2]. Notwithstanding, despite the last novel and advantageous therapeutical modalities, sometimes patients present clinical relapses and fatal complications. Some of this group remain symptomatic despite adequate therapy [3,4]. High concentrations of anti- AChR or anti-MuSK antibodies through radioimmunoassay, Cell-based Assays (CBA), and Enzyme Linked Immuno Assay (ELISA) confirm the MG diagnosis. When patients presenting muscle weakness and AChR or MuSK antibodies are not confirmed by the before-mentioned investigations, they could be grouped as "double seronegative", and the final diagnosis should be confirmed through Nerves Conduction Velocity Test (NCV) and Single- fibre Electromyography (SFEMG) abnormalities due to postsynaptic NMJ disorder.
Myasthenic Crisis (MC) and periods of worsening of the disease are typically seen in this condition which contributes remarkably to the disease burden and keeps the mortality rate around 5%-12% [5,6]. An actual number of MG patients have a poor response to conventional immunotherapies and are named refractory MG (RMG), and anti-CD20 Antibody Rituximab (RTX) seems to be quite beneficial in the management of RMG [7-9]. On the other hand, it has been proved that the pharmacologic spectrum RTX eliminates B cells and its efficacy in cases presenting Refractory MG (RMG) and relapse, mainly in patients receiving systematic infusion every six months [6]. Although should be taken into consideration that repeated infusions lead to harmful immunosuppressive activity [10-11]. Arguably, its activity on B cell homeostasis and the relationship between some B cell subset and the clinical response or relapse. A recent retrospective, a longitudinal study conducted in Mexico, confirmed that RTX improves all clinical manifestations of anti-Acetylcholine Receptor Myasthenia Gravis (anti- AchR MG) and reduces corticosteroid dosage [12]. These authors reported a series of ten patients with positive AchR antibodies RMG with six years median MG duration and poor response to prednisone, azathioprine, and cyclophosphamide but the remarkable improvement to RTX. In the past five years, an increasing number of studies based on a systematic review of MG and RMG have been released into the medical literature [10-33]. Last year, Feng found 16 studies reporting 403 cases of RM treated with Eculizumab (ELX), tacrolimus, cladribine and RTX through a Systematic Review (SR). Their review confirmed that eculizumab and RTX showed the best efficacy and safety results in patients presenting RM. At the same time, ELX caused a more significant adverse event density than RTX (1.195 vs 0.134 per patient-year) [10]. However, other authors reported similar results in 2017, 2018, 2019, and last year [10,12].
During the same period, other authors by SR and Meta-Analysis (MA) of the random-effect model of combined drugs in 1,206,961,907 people determined the prevalence of MG worldwide around 12.4 people (95% CI 10.6-14.5) per 100,000 people. Furthermore, they concluded that Mycophenolate, Plasma Exchange (PE), and Intravenous Immunoglobulin (IGIV) have favourable effects in the treatment of MG [13]. Therefore, it is fear to highlight two other SRs on diagnostic investigations and epidemiology of MG based on its relevant results, done even before [34,35]. Last year, Liu evaluated the efficacy of MG's Double Filtration Plasmapheresis (DFPP) therapy through an SR and MA. They found evidence in 7 randomized control trials and two clinical control trials (329 cases) that DFPP therapy eliminates autoantibodies, reduces AchRab levels, days of hospital stay and QMGS leading to a beneficial effect on MG patients [14]. Between 1997 and 2017, Ipe performed another SR (64 articles) and MA (11 articles) on the efficacy of Therapeutic Plasma Exchange (TPE) and other treatment modalities of MG. They found that TPE provided a higher response rate than IVIG in patients with MG, including those who underwent Thymectomy (Ty) [23].
Convincing results from SR/MA investigations about the beneficial effects of Ty in MG patients have been published recently [16-18]. Thymectomy has been among the most selected topics for SR/MA studies over the past four years [16-18]. The first investigation of this group to evaluate the efficacy of thymectomy compared to medical therapy for non- thymomatous MG in 5841 cases (2930 non-surgical and 2911 surgical) in two RCTs reported better results in patients treated surgically. Four retrospective investigations (379 cases) also confirmed the previous statement (OR 4.10, 95% CI 2.25 to 7.44; I2=20%) with remission rate higher than the non-surgical group [16]. Two years later, other investigators made an SR/MA study to assess the relationship between MC after Ty (MCAT). From 458 identified publications, 25 were eligible for MA. The authors concluded that there are many risk factors for MC after Ty, and the pathogenesis of this process is still far from being confirmed. Last year, other authors for answering the next two research questions: "do patients with Late-Onset Non-Thymomatous MG (LONTMG) obtain the same effects from thymectomy as early-onset cases? and does thymectomy provide any advantage for late-onset NTMG patients?" they performed an SR/MA study searching the medical literature from January 1, 1950, to March 10, 2021, and found that LONTMG cases presented less chance of achieving CSR after Ty compared with the early-onset group if the surgical procedure is done with caution [18]. The last SR investigation on thymectomy was made by Alghamdi and Chen this year. The first one evaluates the effect of Ty on the outcome of thymolipomatous MG in a series of 19 female and 17 male patients. They found that thymoma on CT scans remarkably improved after Ty, mainly in the younger population [35]. The authors in the second research compared the outcomes of Robot-Assisted Thoracic Surgery (RATS) vs Video-Assisted Thoracoscopic Surgery (VATS) in patients with thymoma after TY, confirming that RATS provided better post-surgical recovery compared with VATS [30]. The last SR/MA study related to Ty in MG patients reported one case and confirmed the statements mentioned earlier [36]. Apart from the previous SR/MA published in the medical literature in the past four years, other investigations related to anti-MuSK MG related to Tyrosine Kinase inhibitors, multidisciplinary rehabilitation, the role of microbes such as poliovirus, Epstein-Barr virus, and human papillomavirus in the pathogenesis of MG, and the relationship between MG and COVID-19 reporting that cytokine storm caused by COVID-19 increase the risk of new-onset MG, respiratory failure, MC and the overall mortality rate [20,21,23,37]. On the other hand, other investigators evaluated the same aspects plus the outcomes of invasive ventilator therapy of 152 MG cases with SARS-CoV-2 infections [38].
From 2018 up to date, an important number of investigations evaluating the use of RTX for the treatment of MG reporting good results have been published [9,31,39-47]. Nevertheless, some research questions are still pending answers, such as
1. What percentage of clinical improvement in AChR-MG and MuSK- MG on RTX therapy?
2. What percentage of patient’s present Myasthenia Gravis Exacerbation (MGE) or MC after initiating rituximab during the follow-up period?
3. What is the proportion of Concurrent Immunomodulatory Therapies (CIT)?
4. What is the percentage of adverse responses after initiating RTX.
5. What is the Mortality Rate (MR) in RMG patients under RTX therapy?
The central aid of this study is to answer the previous questions after a systematic review of the available medical literature, including other SR to review the optimal treatments for RMG with a particular focus on RTX therapy.
Material and Methods
We extensively reviewed the available medical literature to answer our research questions.
Eligibility criteriaWe selected publications about patients presenting specific striated muscle weakness, dysarthria/dysphagia, easy fatigability, diplopia/ ophthamoparesis/ophthalmoplegia, typically less pronounced in the morning compared with the evening or after continuous muscular activity followed by improvement after rest. Those patients had any age or gender, and a positive neostigmine test supported the diagnosis of MG, repetitive nerves electrical stimulation test showing decreased more than amplitude after low-frequency stimulation, "trembling" widening during Single Fibre Electromyography (SFEMG) with or without block, and the presence of anti- Musk antibody, anti-LRP4 antibody, plus AChR antibodies on immunological examination. The China medical association in China has recommended the selection mentioned above criteria since 2015 [25]. Apart from RTX, we included other treatment modalities like steroids, pyridostigmine bromide, immunosuppressive medications, other pharmacotherapies, non-pharmacotherapies, and even studies on traditional chinese medicine clinical trials based on TRX within this period.
Literature search methods
We updated the information released by the CADHT report in 2018. Therefore, a limited number of publications were searched on crucial resources, including Medline, Scopus online databases, Google Scholar, Science Direct, Scielo, Search of Sciences, BioRxiv, medRxiv, and the Cochrane Database of Systemic Reviews from January 1, 2018, to July 31, 2022. We did not retrieve some studies by filters. The search of major health technology agencies was also performed to update information published since July 2018. Therefore, we did not restrict the dissemination type if it was reachable. We also selected English, Spanish, and Portuguese publications, unpublished papers, and conference abstracts. We also reviewed the references of each publication included in the SR/MA to identify any missing studies. Some authors were contacted by email to request additional information when it was prudent.
Study and cohort selection and data extraction
Eligibility assessment of the selected publications was performed separately by two authors in an unblinded and standardized process. Two authors reviewed each publication independently and Note Express V3.0 was used. All titles and abstracts were screened, and unsuitable articles were removed. The first author (LFIV) collected data from included RCTs, while another reviewer (SG, HFS) analysed the extracted data. Any disagreements were solved by discussion among authors. We used a data extraction form made for this purpose, including title, list of authors, clinical features of patients, the outcome of patients on RTX, records of patients presenting MG exacerbations or MC, concurrent immunomodulatory therapies, adverse reaction to RTX and mortality rate. When confusing queries arise, we contact the authors to obtain raw data. Again, any disagreement between reviewers was resolved by discussion.
Data collection process
The following data were extracted by one investigator and then crosschecked by the second one: general information concerning the study, study design, main results/findings, and rehabilitation approaches in the experimental group, and the number of participants. Disagreements between the two reviewers were resolved by discussion.
Study risk of bias assessment
We assessed the risk of bias in our selected studies using ROB 2 with an excel tool for symptoms of RMG on RTX as the primary outcome. We included the effect of assignment to intervention, randomization process, analysis of the outcome, missing outcome information, overall assessment, and selection of the reported result. Both authors assessed the risk of bias separately, and after discussion, they solved any disagreement. If any investigation did not show the correct randomization procedure, the adjusted statistical process for deviations such as ITT analysis we considered that published trial did not use the appropriated method to estimate the effect of assignment to intervention and was excluded. If the studies of Randomized Control Trials (RCT) did not publish their protocol, we did not recognize evidence for the missing outcome information to confirm the existence of drop-out. If we did not find evidence of blindness of the assessors in a hospital environment outcome evaluation, then we did not include that report. If the risk of bias increases under any circumstance, we agreed that the overall evaluation is "high risk" with or without the "high-risk domain."
Effect measures
The continuous data and binary data related to outcome, CIT, MR, and side effects of RTX were presented as Mean Difference (MD) at 95% Confidence Interval (CI) and Risk Ratio (RR) at the same CI for the curative and total effective rate. Due to the clinical heterogeneity, the random effect was used for MA when it was highly required because the primary aid of this study is more SR than MA. Nonetheless, the information obtained from RTX therapy under different dosages was compared with different kinds of conventional therapies, and the MA was performed on the results, the effective rates, RMG clinical scores, and secondary outcomes and results delivered by MD (95% CI) and the effective rates into RR (95% CI).
Selection criteria
All parameters used for selecting the data are detailed below. As previously mentioned, both authors were equally involved in screening and selecting manuscripts. In the beginning, only titles and abstracts were reviewed, and all the relevant papers were assessed and retrieved for the final selection based on the inclusion criteria to be shown below in Table 1.
| Criteria | Description |
|---|---|
| Population | All patients diagnosed with myasthenia gravis based on clinical criteria and/or laboratory confirmation no responding to the standard dosage of RTX or reactive to tolerate it. |
| Intervention | G1: RTX: induction therapy (initial course) G2: Re-indication of RTX: in case of flares |
| Comparator | G3: RTX: maintenance treatment despite of initial response G4: RTX: as previous modalities (1,2,3) G1 and G2: standard therapy (e.g., corticosteroids, plasma exchange, IV immunoglobulin, cyclosporine, cholinesterase inhibitors, methotrexate, azathioprine, thymectomy, cyclophosphamide, obinutuzumab, tacrolimus, ecolizumab); no therapy; no correlation. G3: Repeated Tx with RTX because RMG flare or relapse; standard therapy upon disease flare/relapse; no therapy; no correlation. |
| G4: Any correlation for the treatment of MG. | |
| Outcomes | G1 to G3: Effectiveness of Tx (e.g., adverse events, remission, clinical response, need for steroids, immunotherapy or plasmapheresis, investigations results, quality of life) G4: Cost per quality-adjusted life-year gained. |
| Study | Systematic reviews, MA, health technology assessments, non-randomized studies. |
Exclusion criteria
All manuscripts unsuitable to be included in the previous table were removed. In addition, the publications screened by Calvin were also excluded, articles published before 2018, duplicate reports, papers with incomplete results, and unclear methodology [39].
Patient population
The SR with MA included studies of patients with refractory anti-AChR antibody-positive MG. A total of 397 patients were included in the review. The mean age of patients at the time of treatment was 41.9 (43.7) years (median=42.6 years), the proportion of female patients was 66%, and the mean disease duration at the time of treatment was 43.44 months. All non- randomized studies included cases with MG refractory to corticosteroid or presented unusual adverse reactions to conventional treatment [9,38-48]. The number of relevant patients in each study ranged between 5 and 71, and the number of cases selected in all non-randomized studies was 191 (47 were included in the systematic review with meta-analysis). Nine studies were specific to adult populations, and only one was specific to children and adolescents [44]. In one study, it was not well clarified if children and adults were included or if the total population only included adults. The mean or median ages of patients included in the non-randomized studies ranged between 11.6 and 65.3 years [41]. The proportion of female patients varied between 10.2% and 96.7%. The mean or median disease duration at the treatment time ranged between 13.4 months and 192.0 months. Although the definitions of low dose and routine dose of RTX were not explained in these manuscripts, both treatment modalities were included in all selected SR and MA. Therefore, the dosage of RTX infusions varied across the investigations. The most common dosage of RTX used was 375 mg/m2. However, some patients received fixed doses of 500 mg, 600 mg, 720 mg or even 1000 mg/m2.
It is essential to highlight that most investigations included patients with re-treatments or therapy maintenance due to their outcomes. The dosage of RTX and type of maintenance therapy were not equal in all publications; therefore, we added different analytical procedures accordingly. All studies did not include control groups and only accepted non-randomized patients on RTX. All treating physicians selected the most convenient therapeutical protocol separately, including induction doses, re-treatment, or maintenance; therefore, we assess the clinical outcomes, presence of MGE or MC, and the characteristic of associated immunomodulatory treatment results separately adverse response and even death.
Synthesis method
To provide an adequate sensitive analysis, we select studies assessing the outcome of blindness procedures and those related to clinical improvement (AChR-MG and MuSK-MG) on TRX, data containing data on MGE/MC after initiation of RTX therapy, the proportion of CIT, side effects, and mortality rate during the period of treatment. The previously mentioned manuscripts were eligible for MA if their results were stable without a placebo effect. To assess the chances of publication bias, we used a funnel plot, as has been recommended Page [43].
Results
One recent study of SR/MA on this issue included RCT and uncontrolled observational investigations delivered between January 1, 2008, and January 30, 2020 [49]. Their authors found 21 relevant investigations suitable for this report. All research was single-arm observational and uncontrolled studies. From the total of non-randomized studies (n=11) only two were prospective single-arm cohort investigations, and the rest were, single-arm cohort retrospective studies without control group [9,38-49]. Six studies were performed in single institutions and three of them in multicentre [40,45,47].
Results of the search
Twelve publications about RTX therapy in MG and RMG were identified, and twenty-eight other investigations were made under SR/MA process [10,15-37]. A total of 469 investigations were searched from the selected sites. After 343 duplicates were removed, 126 manuscripts were identified. Additional 71 papers were eliminated after screening all titles and abstracts then 55 full-text versions of publications were assessed. However, 25 records did not meet the criteria to be included and were separated for reasons. Finally, 30 studies were suitable for final selection, as seen in the PRISMA flow diagram of selected studies (Figure 1).
Study characteristics
All trials were published from 2018 to 2022 in peer-review medical journals, excerpting two that were communicated by dissertations. All studies used diagnosis criteria from the Chinese medical association neurology branch Guidelines for diagnosing and treating MG [50].
Comments and final remarks
One of the aids of this study is to report the clinical effectiveness and cost-effectiveness of RTX therapy for MG cases refractory to standard treatment according to the medical literature reviewed and we found no evidence on the cost-effectiveness of RTX for the treatment of RMG [48].
Rituximab
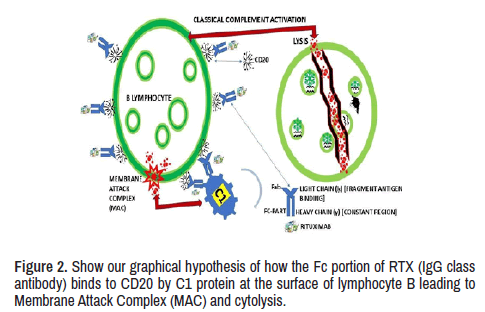
We define RTX as a humanized chimeric monoclonal antibody directed to CD-20, leading to complement-mediated cytotoxicity which cause depletion of CD-20+ cells, preventing proliferation and activation of lymphocyte B. RTX can be identified as an IgG class antibody with an Fc portion able to bind to CD20 on the surface of B-cell by C1 protein. We support that this protein activates the classical component cascade leading to the formation of Membrane Attack Complex (MAC) and cytolysis, as it has been shown in Figure 2. Based on our findings after SR of the medical literature on RTX and AChR antibodies MG and even MuSK RMG, we have hypothesized one mechanism of action of RTX on NMJ postsynaptic disorder, which is graphically represented in Figure 2.
Clinical effectiveness of RTX
This report seeks to answer three research questions related to the effectiveness of RTX induction therapy, RTX re-treatment, and RTX maintenance therapy for managing MG. Only one SR and seven non-randomized studies reported on the benefits and safety of RTX. Unfortunately, the medical literature did not assess the effectiveness of RTX used as we planned and only considered exclusively in one of these three manners. Most reports refer to cases that received an initial course of RTX [9,40,41,44-47,49]. The subsequent dosages or re-treatments were introduced according to the patient clinical response, as we will discuss below.
As previously cited, the dosage of RTX and the frequency of its administration differed from one publication to the next and even within the same investigation, which impeded us from analyzing the reported results from RTX therapy following the same pattern of statistical research. One of the most relevant responses to the therapy according to their SR with MA has been reported by Li, among others [9,38-47,49]. These results came across from 260 cases included in 21 basic investigations under SR/ MA, which reported most of the cases (77.0% (95% CI, 70.1% to 82.6%; P=0.0001)) achieved improved clinical manifestations while were treated with RTX during the median duration of 37.5 months from baseline to follow-up. However, even though an actual number of patients (50.8%) got some improvement, the overall assessment showed results not statistically significant (p=0.921). On the other hand, other authors studied 29 RMG patients by the non-randomized procedure under RTX therapy and found a substantial number of cases achieving remarkable improvement (86.2%) after six months of therapy and even more significant after 12 months of continued RTX treatment (90.5%). In conclusion, comparing the mean muscle score of MG patients before RTX therapy and six months later, the clinical improvement found was statistically significant (p<0.0001) and after twelve months of treatment, even better (p=0.006) [40].
In 2020, Litchman and collaborators selected 33 anti-AChR and anti- MuSK positive MG patients in a single-centre retrospective study looking for their response to RTX. They informed that 63.6% of cases achieved clinical remission, and 48.5% of their series relapsed during the follow-up period (1,861 days) [41]. During the same year, Zhong and colleagues studied a small group of patients (n=12) and reported a statistically significant diminishing of remarkable response in mean MG-specific manual test results (67.4% decrease; p=0.019) after six months of therapy compared to baseline parameters [43]. At the same time, Zingariello also reported a decreased mean quality response in their five patients, but they did not perform statistical analysis to prove it [44]. During the same period, another small series (n=9) was assessed by Marino. However and now these authors reported that most of their cases (66.6%) achieved excellent responses and stayed away from intravenous immunoglobulin (IVIG) or plasmapheresis to reduce corticosteroids doses and withdrawal immunosuppressors. Two patients had partial clinical improvement without reaching the status of minimal clinical manifestations after reducing 50% of corticosteroids and excluding IVIG, plasma exchange and immunotherapy. Only one case showed no response. In 2019 and following the same evaluating clinical procedure, Choi and collaborators selected 17 MG patients to determine the efficacy of RTX during a median time of 7.6 months. They found 11 cases (65%) responding well to the treatment despite receiving only ≤ 5 mg of prednisone per day during the follow-up period [45]. Simultaneously, Jing and collaborators assessed clinical response in 15 cases of RTX at the baseline stage and six-month post-RTX and found that mean values diminished remarkably from 15.7 (SD=4.9) to 11.2 (SD=4.4; P=0.013) [46]. Notwithstanding, other research was done under similar circumstances, and they did not follow the same protocol. Similar results have been reported by Singh and Goya (n=8) and Topakian (n=56) Despite these authors did not perform statistical analysis [9,47].
Systematic reviews
This SR/MA is based on the previous clearly defined selection/ exclusion criteria; it is orientated to answer the research questions, uniform searching of multiple databases on key terminology (MG/RTX), and limited restrictions such as publications made in English, Spanish and Portuguese and restricted to the period of five years of publications. The methodology for selecting publications and data extraction was strictly documented, and all selections made by one author were reviewed by the other based on the previous experience of other authors [49]. Results were graphically represented following the PRISMA guidelines (Figure 1), illustrating reasons to exclude manuscripts and the selection process. Like most authors, we did not receive funds during this SR; therefore, funding sources were not disclosed. Most authors did not explain the criteria used for their eligible study design (RCT or human research), and we did the same. Nobody releases a list of excluded publications after their full-text review, and we follow the same procedure to provide better uniformity in delivered results. We reached the same level of risk for the lack of capturing relevant non- indexed papers during our searching process as other authors because we did not include grey literature. On the other hand, we also declare an elevated risk for selective reports because we did not separate the review method from the rest of the process at the beginning. It was not described in the protocol of other investigators either. A critical methodological limitation of our SR was an incomplete assessment of the quality or risk of bias for each primary study, as happened to other investigators leading to a lack of proper interpretation and discussion of the results of primary studies (review and potential impact of risk of bias). The quality of studies in SR was low due to deficiencies in the study design, such as uncontrolled single-arm cohort investigations [49].
Outcomes
Trying to answer the first research question, we selected several cases that achieved a definite improvement in motor function, including strength and tone of several muscles (extraocular, bulbar, respiratory and extremities), clinical remissions, relapsing period, MG American foundation (MGAF), quality MG scores (QMG-13-item scale used to quantify severity involvement in MG), decrease severity and changes of localized symptoms, and any other changes between complete stable remission to a fatal outcome. Most studies scored each striate/extraocular muscle contraction from 0 (average) to 4 (paralysis), and the sum of each muscle score represented the total score which ranged between 0 (standard muscle power) to 72. Total scores range between 0 and 39 (maximal myasthenic weakness). Most studies revealed lower total scores (mean: 9) when patients received TRX at a therapeutic dosage.
Concurrent immunomodulatory therapies
Recently, some authors have reported changes to CIT in the medical literature. They included steroids, prednisone, oral steroid-sparing agents in their SR [49] and eight non-randomized investigations [9,40-42, 44-49]. Most relevant outcomes have been reported from patients achieving the ability to the dosage of prednisone below 10 mg daily or to reduce their dosage up to 50% or even less [44,45], patients able to stop taking steroids medications or any other immunomodulatory agent [9,40,41,47,49] or patients able to tolerate quickly the symptoms taken minimal dosage of immunomodulatory therapy, decreased frequency of IVIG and PE [9,41,44-47]. Based on our personal experience and the information obtained from this SR, it is possible to highlight the role of RTX therapy on MG patients under CIT, improving their clinical manifestation and providing the capacity to reduce/discontinue the CIT.
Myasthenia gravis exacerbation or myasthenic crisis
Only in four non-randomized investigations have MGE been reported [9,40,41,44] and the MGE and MC in another two publications [9,40]. While MG patients on RTX were admitted to the hospital due to MGE reported by two authors [41,44]. However, Quality of Life (QOL) assessing the mean MG activities of daily living (MG-ADL) scores and 15-item MG-QOL scores were investigated by another two authors [43,46]. The MG-ADL assesses the ability to talk, swallow, breathe, oculomotor function (diplopia/palpebral ptosis), self-care, and other physical activities. Each activity is scored from 0 to 3, and the total score is from 0 to 24, where the higher score represents the major impediment to performing ADL. The 15-item MG-QOL checks 15 aspects divided into four groups related to symptoms, mobility, emotional well-being, and general contentment. Each group is scored from no impairment (0) to too much impaired (4) The sum of all scores determines the total score from highest QOL (0) to much impaired (60). The publication made by Zhong and collaborators on a measure of QOL (n=12) after six months of RTX therapy confirmed a remarkable improvement seen in the mean MG-ADL scores (p=0.022) compared to baseline. However, these authors did not find statistically significant differences (p=0.13) in mean MG-QOL 15 scores [43]. Other authors reported statistically significant improvements in mean MG-ADL scores (P=0.002) and meant MG-QOL 15 scores (P=0.018) in 15 MG cases treated with RTX at six-month follow-up compared to the baseline stage [46]. Unfortunately, the lack of information from different reports impeded the calculation of the exact percentage of patients under RTX therapy presenting MGE/MC during the follow-up, but it seems to be very low.
Adverse response to RTX and mortality rate
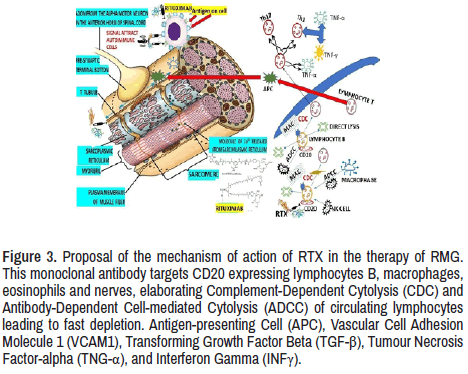
As we before-cited, RTX provides a good response in most patients reported in the medical literature. The mechanism of action is still not well confirmed in RMG cases. Nevertheless, many researchers agreed that RTX acts on B lymphocytes, causing cytolysis of lymphocyte B after binding of RTX to the CD 20 and supported by NK cells, interleukins, TNFα, INFα, interferon, and other immunological cells. The leading role played by the antinuclear antibodies (RTX and others) is to destroy the antigens at the neuromuscular junction to facilitate better neurotransmitter conduction through the postsynaptic receptor leading to better muscle contraction, as we hypothesized in Figure 3.
Figure 3. Proposal of the mechanism of action of RTX in the therapy of RMG. This monoclonal antibody targets CD20 expressing lymphocytes B, macrophages, eosinophils and nerves, elaborating Complement-Dependent Cytolysis (CDC) and Antibody-Dependent Cell-mediated Cytolysis (ADCC) of circulating lymphocytes leading to fast depletion. Antigen-presenting Cell (APC), Vascular Cell Adhesion Molecule 1 (VCAM1), Transforming Growth Factor Beta (TGF-β), Tumour Necrosis Factor-alpha (TNG-α), and Interferon Gamma (INFγ).
Looking for the answer of the question 4, we tried to calculate the percentage of patients presenting side effects or any other adverse response to RTX after initiating the therapeutic program and we found several articles reporting any side effect while other few publications only report specific adverse events such as arrhythmia (3.7%) and (4.5%), infection (24.4%) and (12.9%), cytopenia (5.3%), infusion reaction (7%), (11.8%) and (19.2%), psychiatry disorders (3.7%), and death [9,37-50]. Other types of adverse reaction like herpes zoster (n=1) (5.9%) from 17 participants, transaminitis (n=3) from A total of eight of patients, osteonecrosis (n=1), hepatitis B (n=1) and respiratory tract infections (n=3), enteritis (n=2), erysipelas (n=1), cholecystitis (n=1), chronic pain syndromes (n=2), herpes zoster (n=1), an unspecified mental disorder (n=1), and alopecia areata (n=1) from a series of n=56. Considering the results from the most extensive retrospective study on adverse events associated with RTX therapy, we can affirm that those undesired effects were relatively common, and the reported frequency is between 25% to 45%. However, no dangerous signs were confirmed, and RTX remains an excellent therapeutic choice for both MuSK+ and AChR+ generalized MG, mainly for patients presenting poor disease control [48]. Unfortunately, we did not find any RCT comparing the effectiveness of other therapies over RTX, and most of the investigations did not include a control group in their statistical analysis.
In this publication, we summarize the findings of authors treating RMG patients with RTX, including the results of CADTH in 2018. The SR/MA made by Li (n=260) last year reported 26.4% of adverse events included pooled data from the included 19 primary investigations, while other authors found it in 42% of their series (n=28) [44]. In addition, the mortality rate was reported from 5.9% to 7.5% of cases, but this event occurred at different lengths of the follow-up period [45,49]. Other authors like Singh and Goyal included eight patients treated with rituximab [9]. None of the patients had infusion-associated reactions or cytopenia post-rituximab infusion. Topakian, stated that RTX was generally well-tolerated by the 56 patients from their series; however, several side effects and complications potentially related to rituximab were observed during follow-up (median duration of 20 months), including infusion reactions (n=3), respiratory tract infections (n=3) [48]. One 59-year-old patient died 4.5 months after starting rituximab. The cause of death was assumed to be related to cardiac complications, although post-mortem investigations did not confirm it.
Conclusion
Our SR of the medical literature confirmed the improvement of symptoms and signs associated with MG/RMG after initiating RTX therapy. This efficacious therapy provides a better QOL in most cases treated and contributed to beneficial outcomes in almost all cases reported in the medical literature. This SR also confirmed that RTX is well tolerated in both conditions (AChR-MG and MuSK-MG) almost equally. The cases presenting any adverse event after initiating RTX ranged from 26.4% and 42.8% and never were considered severe complications. Patients presenting AChR- RMG also improve when receiving repeated lower doses of RTX. However, multi-centre RCT using different doses of RTX in an extensive series should be performed to confirm its efficacy. The frequency of MGE/MC after initiating RTX therapy has not been determined. Incidence/prevalence of mortality has been no reported and results from post-mortem examinations were not published up to date. Unfortunately, no evidence-based guidelines and relevant cost-effectiveness have been demonstrated. Apart from the clinical benefits obtained with RTX, a substantial number of patients were able to reduce doses and frequency of associated immunomodulatory agents.
Limitations
We afford some limitations with the selected articles due to small series, elevated risk of bias, lack of comparative groups, mixed clinical groups without well-designed protocol, the inclusion of single-arm cohort studies, unclear criteria for selection/exclusion process, lack of high-quality investigations and RCT comparing the placebo and other modalities of treatment. In addition, most of the publications were retrospective in design. We also found a lack of uniformity when manual muscle tests, MG-QOL 15 scores and QMG scores reported by some investigators were processed. Unfortunately, we could not contact the authors for clarification.
Future Research
Several limitations must be highlighted regarding this research. First, the research design adopted in this study was cross-sectional, and data were collected at a single time. Whereas, to generalize the findings of a similar study in the future, the researchers should employ a longitudinal research design. Second, the present research used a convenience sampling approach in this study. Future scientists can adopt any probability sampling approach to reach their respondents. Third, this study used a survey questionnaire to gather data from respondents. It will be interesting to adopt the mixed methodological approach in future similar studies. In the end, the underpinning theory used in this research is SOR Model by Mehrabian and Russell (1974). Whereas, the TAM model can also provide a different insight from a similar research model.
Contributions
Practical contribution
This study has both theoretical and practical contributions. In terms of practical contribution, this study provides a mechanism for the artists and painters by which they can improve their mental health. This study provides the way forward to the usage of metaverse technology and its benefits. The virtual world can help painters to improve their aesthetic awareness. Aesthetic awareness is very important as the painters and artists create a point of difference in their aesthetic abilities. Also, the virtual world can help artists to improve their social life. They can remain connected to their clients and family members through the virtual world. In the presence of improved social life and aesthetic awareness, the mental health of the painters will be improved. Mental health is an issue of concern in almost every industry. Therefore, it is very important to study the factors that can help in improving the mental health of the painters. The findings of the study help make policies and strategies to improve the mental health of almost every professional.
Theoretical contribution
The main objective of the present study was to examine the effect of metaverse-based painting performance on mental health, social connectedness, and aesthetic awareness. This research also examined the mediating role of aesthetic awareness and social connectedness. This study bridges the gap of limited studies that examined the role of the virtual world in quest to improve the mental health. Also, there is a dearth of knowledge that can explain the role of metaverse technology to improve the social networking and aesthetic awareness of painters. This study has provided new insight regarding the role of metaverse-based technology in this scenario. Also, in terms of the SOR model Mehrabian and Russell (1974), this study has examined the Metaverse-based technology as Stimulus (S) first time in literature till date.
Declaration
Ethical issue and consent to publish
We certify that we did not disclose any identity issues of any patient included for analysis and the patient's anonymity is guaranteed.
Availability of data
Data used in this study are available on reasonable request from the corresponding author.
Competing interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
We did not receive funds or any contribution to this publication.
Author's contribution
All authors made equal contributions to the elaboration of this manuscript. HFS searched Medline by PubMed, Google Scholar, Science Direct, Scopus, and LdeFIV searched Embase, Scielo, LILACS, BIREME, Cochrane library and WHO database. Both authors collected all patient information and planned this report; LdeFIV and SSJ wrote the first draft. HFS wrote the final draft. All authors have read all written drafts and agreed to the published last version of this article
Acknowledgment
Special thanks to all medical interns rotating through the division of neurology (NMACH/WSU) for their kind and enthusiastic participation identifying some articles reported in other publication sources.
References
- Gilhus, Nils Erik, Socrates Tzartos, Amelia Evoli and Jacqueline Palace et al. "Myasthenia gravis." Nat Rev Dis Primers 5 (2019): 30.
- Dalakas, Marinos C. "Immunotherapy in Myasthenia Gravis in the Era of Biologics." Nat Rev Neurol 15 (2019): 113-124.
- Mantegazza, Renato and Carlo Antozzi. "When Myasthenia Gravis is Deemed Refractory: Clinical Signposts and Treatment Strategies." Ther Adv Neurol Disord 11 (2018).
- Schneider-Gold, Christiane, Tim Hagenacker, Nico Melzer and Tobias Ruck. "Understanding the burden of refractory myasthenia gravis." Ther Adv Neurol Disord 12 (2019): 1-16.
- Alshekhlee A, Miles JD, Katirji B and Preston DC et al. "Incidence and Mortality Rates of Myasthenia Gravis and Myasthenic Crisis in Us Hospitals." Neurology 72 (2009): 1548-1554.
[Crossref] [Google Scholar] [Pubmed]
- Neumann, Bernhard, Klemens Angstwurm, Philipp Mergenthaler and Siegfried Kohleret al. "Myasthenic Crisis Demanding Mechanical Ventilation: A Multicenter Analysis of 250 Cases." Neurology 94 (2020): e299-e313.
[Crossref] [Google Scholar] [Pubmed]
- Jing, Sisi, Yang Song, Jie Song and Song Panget al. "Responsiveness to Low-Dose Rituximab in Refractory Generalized Myasthenia Gravis." J Neuroimmunol 311 (2017): 14-21.
- Afanasiev, Vadim, Sophie Demeret, Francis Bolgert and Bruno Eymard et al. "Resistant Myasthenia Gravis and Rituximab: A Monocentric Retrospective Study of 28 Patients." Neuromuscul Disord 27 (2017): 251-258.
[Crossref] [Google Scholar] [Pubmed]
- Jing S, Lu J, Song J and Luo S et al. "Effect of Low-Dose Rituximab Treatment on T- and B-Cell Lymphocyte Imbalance in Refractory Myasthenia Gravis". J Neuroimmunol 332 (2019): 216-223.
- Xuelin, Feng, Zubiao Song, Mengli Wu and Yanmei Liu et al. "Efficacy and Safety of Immunotherapies in Refractory Myasthenia Gravis: A Systematic Review and Meta-Analysis". Front Neurol 12 (2021): 725700.
- Cardinal, Landon Oceanea, Diane Friedman, Marguerite Guiguet, and Pascal Laforêt et al. "Efficacy of Rituximab in Refractory Generalized anti-AChR Myasthenia Gravis." J Neuromuscul Dis 5 (2018): 241-249.
- López-Hernández, Juan Carlos Sr., Javier A Galnares-Olalde, Enrique Gómez-Figueroa, and Adib Jorge de Sarachaga et al. "Rituximab in Refractory Myasthenia Gravis: Experience in a Single Healthcare Center in Mexico." Cureus. 13 (2021): e13226.
- Nader, Salari, Behnaz Fatahi, Yalda Bartina and Mohsen Kazeminia, et al. "The Global Prevalence of Myasthenia Gravis and the Effectiveness of Common Drugs in its Treatment: A Systematic Review and Meta- Analysis." J Transl Med 19 (2021): 516.
- Chaoying, Liu, Peng Liu, Mei Ma and Hongxia Yang et al. "Efficacy and Safety of Double-filtration Plasmapheresis Treatment of Myasthenia gravis. A Systematic Review and Meta-analysis." Medicine (Baltimore) 100 (2021): e25622.
- Abbas, Alzhraa Salah, Nicole Hardy, Sherief Ghozy and Mahmoud Dibas et al. "Characteristics, treatment, and outcomes of Myasthenia Gravis in COVID-19 patients: A systematic review." Clin Neurol Neurosurg 213 (2022): 107140.
- Antônio JM, Cataneo, Gilmar Felisberto Jr., and Daniele C. Cataneo. "Thymectomy in Nonthymomatous Myasthenia Gravis, Systematic Review, and Meta-Analysis." Orphanet J Rare Dis 13 (2018): 99.
- Chaoying, Liu, Peng Liu, Xiao jing Zhang and Wen Qian Li et al. "Assessment of the Risks of A Myasthenic Crisis after Thymectomy in Patients with Myasthenia gravis: A Systematic Review and Meta-Analysis of 25 Studies." J Cardiothorac Surg 15 (2020): 270.
- Jinwei, Zhang, Yuan Chen, Hui Zhang and Zhaoyu Yang et al. "Effects of Thymectomy on Late- Onset Non-Thymomatous Myasthenia gravis: Systematic Review and Meta-Analysis. Orphanet J Rare Dis 16 (2021): 232.
- Waqar, Waheed, Eric Newman, Marwa Aboukhatwa and Maryam Moin et al. "Practical Management for Using Eculizumab in the Treatment of Severe, Refractory, Non-Thymomatous, Achr+Generalized Myasthenia gravis: A Systematic Review." Ther Clin Risk Manag 18 (2022): 773-774.
- Victoria, Leopardi, Yu-Mei Chang, Andrew Pham and Jie Luo et al. Garden. "A Systematic Review of the Potential Implication of Infectious Agents in Myasthenia gravis". Front Neurol 12 (2021): 618021.
- Dimitrios C, Ziogas, Dimitrios Mandellos, Charalampos Theocharopoulos and Panagiotis-Petros Lialios et al. "Neuromuscular Complications of Targeted Anticancer Agents: Can Tyrosine Kinase Inhibitors Induce Myasthenia Gravis? Getting Answers from a Case Report up to a Systematic Review". Front Oncol 11 (2021): 727010.
- Tina, S Ipe, Adeola R Davis, Jay S Raval. "Therapeutic Plasma Exchange in Myasthenia Gravis: A Systematic Literature Review and Meta-analysis of Comparative Evidence." Front Neurol 12 (2021): 662856.
[Crossref] [Google Scholar] [Pubmed]
- Bruno, Corrado, Benedetto Giardulli, Massimo Costa. "Evidence-Based Practice in Rehabilitation of Myasthenia Gravis. A Systematic Review of the Literature." J Funct Morphol Kinesiol 5 (2020): 71.
- Zeead M, Alghamdi, Sharifah A Othman, Mohammed Sabry Abdelmotaleb and Farouk Alreshaid et al. "Thymolipomatous myasthenia gravis outcomes following thymectomy: A systematic review." Interact Cardiovasc Thorac Surg. 34 (2022): 584-589.
- Si-jia, Zhu, Rui-ting Wang, Ze-yu Yu and Ruo-xiang Zheng, et al. "Chinese Herbal Medicine for Myasthenia Gravis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials." Integr Med Res 11 (2022): 100806.
- Zuojie, Zhang, Chunsong Yang, Lingli Zhang and Qiushi Yi et al. "Efficacy and Safety of Tacrolimus in Myasthenia Gravis: A Systematic Review and Meta-analysis." Ann Indian Acad Neurol 20 (2017): 341-347.
- Shuang, Chen, Meng-Bei Xu, Xiao-Li Zhou and Pei-Qing Rong et al. "Chinese Herbal Medicine for Myasthenia Gravis: A Systematic Review and Meta-Analysis". Front Pharmacol 9 (2018): 969.
- Harrison, Banner, Kirsten M Niles, Michelle Ryu and Mathew Sermer et al. "Myasthenia Gravis in Pregnancy: Systematic Review and Case Series." Obstet Med 15 (2022): 108-117.
- Jiayu, Shi, Ying Tan, Yangyu Huang and Ke Li et al. "Association Between Clinical Factors and Result of Immune Checkpoint Inhibitor Related Myasthenia Gravis: A Single Center Experience and Systematic Review." Front Neurol 13 (2022): 858628.
- Cheng, Shen, Jialong Li, Jue Li and Guowei Che. "Robot‐Assisted Thoracic Surgery versus Video‐Assisted Thoracic Surgery for Treatment of Patients with Thymoma: A Systematic Review and Meta‐Analysis." Thorac Cancer 13 (2022): 151-161.
- Srabani, Banerjee and Lorna Adcock. "Rituximab for Treating Myasthenia Gravis: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines."
[Google Scholar] [Pubmed]
- Mingxia, Li, Fangfang Ge, Rongjing Guo and Zhe Ruan et al. "Do Early Prednisolone and Other Immunosuppressant Therapies Prevent Generalization in Ocular Myasthenia Gravis in Western Populations: A Systematic Review and Meta-Analysis." Ther Adv Neurol Disord 12 (2019): 1756286419876521.
- Michael, Benatar. "A systematic review of diagnostic studies in myasthenia gravis." Neuromuscul Disord 16 (2006): 459-467.
- Aisling, S Carr, Chris R Cardwell, Peter O McCarron and John McConville. "A Systematic Review of Population-Based Epidemiological Studies in Myasthenia Gra-Vis." BMC Neurol 10 (2010): 46.
- Zeead, M Alghamdi, Sharifah A Othman, Mohammed Sabry Abdelmotaleb and Farouk Alreshaid et al. "Thymolipomatous Myasthenia Gravis Outcomes Following Thymectomy: A Systematic Review." Interact Cardiovasc Thorac Surg 34 2022: 584-589.
- Louise, Gurowich, Adam Maxwell, Alexandra Rice and Gabriel Yiin. "Correction: Post-Thymectomy Myasthenia Gravis: A Case Report and Systematic Literature Review." BMJ Case Rep 15 2022: e246005corr1.
- Dodik, Tugasworo, Aditya Kurnianto, Retnaningsih and Yovita Andhitara et al. "The Relationship between Myasthenia Gravis and Covid-19: A Systematic Review." Egypt J Neurol Psychiatr Neurosurg 58 (2022): 83.
- Alzhraa, Salah Abbas, Nicole Hardy, Sherief Ghozy and Mahmoud Dibas et al. "Characteristics, Treatment, and Outcomes of Myasthenia Gravis in Covid-19 Patients: A Systematic Review." Clin Neurol Neurosurg 213 (2022): 107140.
- Calvin, Young and Sarah C McGill. Rituximab for the Treatment of Myasthenia Gravis: A 2021 Update. Can J Health Technology 2021.
- Jonathan, AC Sterne, Jelena Savović, Matthew J Page and Roy G Elbers et al. "RoB 2: A Revised Tool for Assessing the Risk of Bias in Randomized Trials." BMJ 366 (2019): l4898.
- Dos, Santos A, Noury JB, Genestet S and A. Nadaj‐Pakleza et al. "Efficacy and Safety of Rituximab In Myasthenia Gravis: A French Multi-Centre Real-Life Study." Eur J Neurol 27 (2020): 2277-2285.
- Tess, Litchman, Bhaskar Roy, Aditya Kumar, and Aditi Sharma et al. "Differential Response to Rituximab in Anti-Achr and Anti-Musk Positive Myasthenia Gravis Patients: A Single-Centre Retrospective Study." J Neurol Sci 411 (2020): 116690.
- Mariapaola, Marino, Umberto Basile, Gregorio Spagni and Cecilia Napodano et al. "Long-Lasting Rituximab-Induced Reduction of Specific-but not Total-Igg4 in Musk-Positive Myasthenia Gravis." Front Immunol 11 (2020): 613.
- Huahua, Zhong, Jun Lu, Sisi Jing and Jianying X et al. "Low-Dose Rituximab Lowers Serum Exosomal Mir-150-5p In Achr- Positive Refractory Myasthenia Gravis Patients." J Neuroimmunol 348 (2020): 577383.
- Zingariello CD, Elder ME, Kang PB. "Rituximab as Adjunct Maintenance Therapy for Refractory Juvenile Myasthenia Gravis." Pediatr neurol 111 (2020): 40-43.
- Kyomin, Choi, Yoon-Ho Hong, So-Hyun Ahn and Seol-Hee Baek, et al. "Repeated Low-Dose Rituximab Treatment Based on the Assessment of Circulating B Cells in Patients with Refractory Myasthenia Gravis." Ther Adv Neurol Disord 12 (2019): 1756286419871187.
- Singh, Nishita and Vinay Goyal. "Rituximab as Induction Therapy in Refractory Myasthenia Gravis: 18-Month Follow-up Study." J Neurol 266 (2019): 1596-1600.
- Topakian, Raffi, Fritz Zimprich, Stephan Iglseder and Norbert Embacher et al. "High Efficacy of Rituximab for Myasthenia Gravis: A Comprehensive Nationwide Study in Austria." J Neurol 266 (2019): 699-706.
- Higgins Julian P.T, James Thomas, Jacqueline Chandler and Miranda Cumpston et al. "In Cochrane handbook for systematic reviews of interventions version 6.2. Assessing the risk of bias due to missing results in synthesis." Cochrane Chapter 13 2021.
- Li, Ting, Guo-Qian Zhang, Yue Li and Shu-An Dong et al. "Efficacy and Safety of Different Dosages of Rituximab for Refractory Generalized Achr Myasthenia Gravis: A Meta-Analysis." J Clin Neurosci 85 (2021): 6-12.
Citation: Valdes, Lourdes de Fatima Ibanez, Sibi Sebastian Joseph and Humberto Foyaca Sibat “Rituximab in Refractory Myasthenia Gravis: A Systematic Review.” Clin Schizophr Relat Psychoses 17 (2023). Doi: 10.3371/CSRP.DLSS.011023.
Copyright: © 2023 Valdes LDI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.