Research - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Potential Effect of Botulinum Neurotoxin A in the Treatment of Induced Benign Prostatic Hyperplasia in Male Rats
Ghazi Faisal Majid1*, Mustafa Ghazi Alabbassi2 and Israa B. Raof32Department of Pharmacy, University of Alkafeel, Baghdad, Iraq
3Laboratory Sciences Department, Mustansiriyah University, College of Pharmacy, Baghdad, Iraq
Ghazi Faisal Majid, Pharmacology and Toxicology Department, Mustansiriyah University, College of Pharmacy, Baghdad, Iraq, Email: ghazifaisal95@yahoo.com
Received: 19-Jul-2021 Accepted Date: Aug 02, 2021 ; Published: 09-Aug-2021
Abstract
Background: Researches carried out in humans, dogs, and rats showed that the injection of botulinum neurotoxin type A into the prostate gland reduce the size and other biomarker that indicate botulinum neurotoxin type A as a new promising treatment for benign prostatic hyperplasia.
Aim of the research: The purpose of the study is to evaluate the effects of botulinum neurotoxin type A in the treatment of induced BPH rat model, comparing it with the most powerful standard treatment available composed of alfuzosin and dutasteride (combination of α1 adrenergic antagonist and 5α reductase inhibitor) for BPH rat model and also to describe the mechanism by which botulinum neurotoxin type A effect prostate growth by focusing on acetylcholine-prostate nicotinic receptor signal role in BPH.
Method: Five groups each of them composed of 10 rats. Normal group administered normal saline IP for 42 days. Induction group induced with BPH by testosterone 4 mg/kg/day SC injection for 14 days then administered normal saline IP for 28 days. Conventional treatment group induced BPH by testosterone 4mg/kg/day SC injection for 14 days then administered combination of alfuzosin and dutasteride daily through oral gavage for 28 days. Botulinum group induced BPH by testosterone 4 mg/kg/day SC injection for 14 days, then the injected inside the prostate at day 15 with botulinum neurotoxin type A and left free for 27 days. Nicotine group induced BPH by testosterone for 14 days, then the injected inside the prostate at day 15 with botulinum neurotoxin type A and administered nicotine orally for 27 days. At the end of the experiment rats weighed and sacrificed and blood and prostate samples were collected for PI, CPV, APV determination and sTNFα, sPSA, sCASP3, pTNFα,pPSA and pCASP3 ELISA testing.
Results: Botulinum, conventional treatment and normal groups had a PI (g/100 g) equal to 0.239 ± 0.056, 0.244 ± 0.025 and 0.208 ± 0.045 respectively and showed a significant (P ≤ 0.05) reduced PI when compared with induction and nicotine groups which had PI equal to 0.317 ± 0.053 and 0.338 ± 0.088 respectively. Botulinum, conventional treatment and normal groups had CPV (mm3) equal to 472.6 ± 62, 468 ± 123.4 and 370.1 ± 100.3 respectively and expressed a significant (P ≤ 0.05)reduced CPV in comparison with induction and nicotine groups which possessed CPV equivalent to 997.9 ± 148.6 and 845 ± 177 respectively. Botulinum, conventional treatment and normal groups had a APV (ml) equal to 380 ± 94.8, 385 ± 127 and 330 ± 67.4 in that order and showed a significant (P ≤ 0.05) reduced APV when compared with induction and nicotine groups which had a APV equal to 825 ± 139.9 and 845 ± 177 respectively. Botulinum, conventional treatment and normal groups had a sTNFα (ng/ml) 84.327 ± 28.063, 99.545 ± 27.78 and 78.2 ± 34.195 respectively and pTNFα (ng/ml) equal to 41.272 ± 6.78, 48.763 ± 27.662 and 37.218 ± 6.415 respectively. Induction group and nicotine group possessed a sTNFα equivalent to 139.236 ± 34.691 and 157.836 ± 24.517 respectively and pTNFα equal to 84.054 ± 67.871 and 95.712 ± 47.056 respectively. Botulinum, conventional treatment and normal groups showed a significant (P ≤ 0.05) reduction in both sTNFα and pTNFα in comparison with induction and nicotine groups. Botulinum, normal groups had a sPSA equal to 0.685 ± 0.264 and 0.629 ± 0.126 respectively showed a significant (P ≤ 0.05) reduction in sPSA when compared with induction, nicotine groups which had a sPSA equal to 1.023 ± 0.423 and 1.328 ± 0.356 respectively. Botulinum, normal groups had a pPSA equivalent to 0.339 ± 0.213 and 0.315 ± 0.164 respectively expressed a significant (P ≤ 0.05) reduction on pPSA in comparison with induction and nicotine groups which possessed a pPSA equal to 0.606 ± 0.394 and 0.65 ± 0.24 respectively. Conventional treatment group had a sPSA equal to 0.886 ± 0.188 and pPSA equal to 0.411 ± 0.197 and showed a no significant difference (P>0.05) in both sPSA and pPSA when compared with normal, induction and botulinum groups, on the other hand showed a significant difference with nicotine group. Results showed no significant difference in sCASP3 (P>0.05) between all goups. Botulinum group express a pCASP3 equal to 10.843 ± 7.731 and showed a significant increase (P ≤ 0.05) in PCASP3 when compared with normal, induction, conventional treatment and nicotine groups which had a PCASP3 equal to 2.38 ± 1.627, 2.151 ± 1.582, 3.319 ± 3.504 and 2.15 ± 1.444 respectively. The PPI of BPH of botulinum, conventional treatment and nicotine groups equal to 71.5%, 66.7% and -19.5% respectively.
Conclusion: BTXA intraprostatic injection found to reduce hyperplasia by induction of apoptosis, reduce inflammation and decrease PSA. It also obvious from the results that BTXA as effective as the conventional treatment and have advantage of less side effects and be single injection instead of daily medication. Nicotine administration reverses the effects of Botulinum neurotoxin type A that’s show the central role of nicotinic receptor stimulation in the pathogenesis of BPH.
Keywords
Benign prostatic hyperplasia • Alfuzosin • Dutasteride • Botulinum neurotoxin type A • Nicotine
Introduction
Benign Prostatic Hyperplasia (BPH) is a disease that mainly affects elderly men [1]. Lower Urinary Tract Symptom (LUTS) risk increased in BPH. LUTS can be divided into irritation symptom and obstructive symptoms [2]. BPH is characterized by increased both epithelial and stromal cells number in the area of the prostate gland. Although pathophysiology of BPH is not fully understood, It may be caused by androgenic stimulation that lead to overgrowth and adrenergic action that lead to contraction of smooth muscle [3,4]. Approximately 30% of human cancers occur as a result of tobacco and other pollutant. It has been found that tobacco smoking is specifically increase inflammation in prostate carcinoma. Nicotine forms up to 1.8% of the dry tobacco weight. IL8 expression can be stimulated in the human neutrophil by nicotine. It also found that tobacco smoker prostate cancer patient had increased expression of cytokine, B cell count and NF-κB in prostate gland [5-7]. The European Association of Urology and the American Urological Association recommended two classes of medication. The first group called α1 adrenergic receptor antagonists and the second group called 5α reductase inhibitors. Combination therapy of two drugs above result in reduction of progression of BPH significantly when compared with one class alone [8]. Over the past years, Botulinum neurotoxin (BTXA) found to have therapeutic effects in many diseases such as Strabismus and cosmetic etc,. [9-11]. BTXA effects on the prostate not restricted to its action on the neuromascular junction, it may be also associated with its effect on the neuro-glandular junction. Intraprostatic injection of BTXA can cause involution in the prostate without any complication like local inflammation and general systemic adverse effects [12]. BTXA is derived from type of bacteria called Clostridium [13]. Pharmacologically active BTXA comprising of a light chain and a heavy chain linked by a disulfide bond. Reduction of this disulfide S-S bond releases the light chain which has metalloprotease action [14]. BTXA then associate with catalytic activity towards SNAP25 (synaptosomal associated proteins 25) that lead to break of SNAR complex resulting in block of the release of the neurotransmitters from nerve ending [13-15]. The affected neurotransmitters by BTXA other than acetylcholine may include norepinephrine and others. BTXA showed effects on growth and dynamic contraction of the prostate [13].The prostate gland is also regulated by parasympathetic system through the pelvic nerve that innervates the major pelvic ganglion and the post ganglionic neurons regulate the prostate gland. Acetylcholine expressed a relaxing effect on the induced prostatic contraction by phenylephrine in isolated prostate tissue from rabbit prostate gland. Studies showed Muscarinic 3 receptor subtype cause contraction in rat prostate tissue [16,17]. Nicotinic receptors found in cell membrane of all mammalian cells. These receptors are ionic channels receptor that found in the nervous system and muscle. Recent researchers found that these receptors also present in other tissue and play a role in many diseases such as prostate cancer. The α5 nicotinic acetylcholine receptors (α5nAChR) have a role in growth of prostate gland. When α5nAChR is activated lead to increase Akt. Akt showed to decrease apoptosis, increase expression of androgen receptor [18,19].
The purpose of the study is to evaluate the effects of botulinum neurotoxin type A in the treatment of induced BPH in rat model, comparing it with the most powerful standard treatment available composed of alfuzosin and dutasteride (combination of α1 adrenergic antagonist and 5α reductase inhibitor) for BPH rat model and also to describe the mechanism by which botulinum neurotoxin type A effect prostate growth by focusing on acetylcholine-prostate nicotinic receptor signal role in BPH
Materials and Method
Experimental animals
50 Adult male Wister rats 250-350 gram purchased from the Iraqi center for cancer and medical engineering research. Animals had kept in the animal house in normal room temperature approximately 21κ and sustained at 12 dark/light cycle with maintenance of free food and water access.
Materials
Testosterone Propionate (MH pharma. Co. LTD. Pakistan), Pure Olive Oil, Alfuzosin (Xatral-Sanofi-France), Dutasteride (Avodart-GSK company- UK), Botulinum neurotoxin vial (HUTOX inj-Huons Global Co.Ltd-South Korea). Nicotine lozenges 2mg (Nicorette® Gum-Johnson & Johnson-USA), xylazine (kepro-Holland) and ketamine (Bremer Pharma GmbH-Germany).
Dose calculation and preparation
Dutasteride and alfuzosin dose calculation and preperation: The dose of dutasteride used 0.044 mg/Kg/day. For conversion of the human dose to rat dose, we multiply human dose with 6.2 (conversion factor according to FDA) [20]. For 70 Kg human aminister 0.5 mg/day, so the dose will be 0.00714 mg/Kg/day. Multiplying the dose by conversion factor between rats and human will give the dose for rats: 0.00714 mg/Kg/day*6.2=0.044 mg/ Kg/day. (Avodart capsules diluted with olive oil to prepare 0.11 mg/ml).
Alfuzosin dose For BPH in 70 Kg human who take 10 mg per day. The dose will be 0.142 mg/Kg/day. Rat dose calculated by Multiply the human dose by the conversion factor. Rat dose=0.142 mg/kg/day*6.2. Rat dose=0.88 mg/kg/day. Alfuzosin diluted with distilled water to prepare stock solution of 2.2 mg/ml.
Testosterone propionate dose and preparation: Testosterone propionate added olive oil to prepare stock solution of 20 mg/ml. The dose for BPH induction was 4 mg/kg/day for 14 days [21].
Botulinum neurotoxin type A (BTXA): The vial was dissolved with 0.9% normal saline to prepare stock solution of 25 unit/ml [22]. Rats anesthetized with 50 mg/Kg ketamine Intra-Peritoneal (IP) injection plus IP injection of 12 mg/Kg xylazine. The lower abdominal hair removed by shaver and the incision area dis-infected with povidone iodine 10% and 70% ethyl alcohol. A lower abdominal small incision about 2 cm was made at the midline and the prostate gland together exposed for injection syringe by remove of the surrounding tissue carefully. Ventral lobe of the prostate gland injected with 5U (0.2 ml) of botulinum toxin stock solution. Care took to inject 2.5 (0.1 ml) into each lobule of the ventral lobe of the prostate. The injection area then cleaned. After that, wound of the skin and wall of abdomen closed using 0/4 PGA suturing and disinfected with povidone iodine 10% [23].
Nicotine preparation and administration: Nicotine solution was prepared by dissolving one lozenge containing 2 mg of nicotine in distilled water to prepare stock solution of 0.25 mg/ml. the dose used was 0.1 mg/Kg/day orally [24].
Experimental design
50 Rats were divided into five groups, each group composed of 10 rats. The first group which is the normal group (Group 1) administered IP 0.5 ml/ kg/day of 0.9% normal saline for 42 day. The second group is the induction group (Group 2) administered testosterone propionate subcutaneous injection 4 mg/Kg for 15 day to induce BPH and then administered IP 0.5 ml/ Kg/day of 0.9% normal saline for 28 day. The third group is the conventional therapy group (Group 3) administered subcutaneous injection of testosterone propionate 4 mg/Kg daily for 15 day to induce BPH. Then rats on this group administered combination therapy of alfuzosin hydrochloride 0.88 mg/Kg/ day and dutasteride 0.44 mg/Kg/day. This combination administered by oral gavage daily for 28 day. The fourth group which is the botulinum group (Group 4) composed administered subcutaneous injection of testosterone propionate 4 mg/Kg /day for 15 day to induce BPH. After that, rats in this group administered intraprostatic injection of 5U into the ventral lobe of the prostate [25,26]. Then rats in this group were monitored for any behavior or other abnormality for 27 day. The fifth group is the nicotine group (Group 5), BPH induced by subcutaneous injection of testosterone propionate 4 mg/ Kg daily for 15 day. Then rats administered intraprostatic botulinum toxin 5U into the ventral lobe of the prostate at day 15. Rats in this group then administered 0.1 mg/Kg/day nicotine orally for 27 days. Rats on all groups had weighed and then, sacrificed by IP injection of 200 mg/Kg phenobarbital [23]. Then the prostate had harvested, weighed and cut into two parts, one part fixed in formaldehyde 10% for histopathological examination and the other homogenized for ELISA testing.
Parameter to be measured
Prostate Index (PI) and Percentage of Prostate Inhibition (PPI) calculated from the formula: PI=(Prostate weight *100)/Rat body weight. Unit of PI=gram/100 gram body weight [21]. PPI of Group X (PPIX)={100- [(MPI.X-MPI.C)/(API.I-API.C)]} *100% [20]. [Where is: MPI.X=Mean PI of X, X can represent conventional treatment or botulinum or nicotine group, MPI.C=Mean PI of normal group, MPI.I=Mean PI of induction group] [20].
Calculated Prostate Volume (CPV) and Actual Prostate Volume (APV): After the prostate dissected a vernier caliper used to detect the length, width and height of the prostate gland and the CPV calculated by the formula CPV=(Length * width * height)/2 [20,21]. APV is calculated using fluid displacement according to Archimedes' principle. The Actual prostate volume is calculated from the formula: (APV)=Final volume-Initial volume.
Plasma ELISA testing for the following marker: ELISA a kit for serum purchased from Sunlong Biotech Co LTD-China. According to the instruction blood collected from heart by 5 cc syringe. Blood allowed clotting by leaving it undisturbed at 25κ for 20 minutes. The clot had been removed by centrifuging at 3,000 rpm for 20 minutes. ELISA test done for serum concentration of the following biomarker: Tumor Necrosis Factor α (TNFα), Prostate Specific Antigen (PSA), Caspase 3.
Prostate tissue homogenate ELISA testing for the following parameter: ELISA a kit for tissue purchased from Sunlong Biotech Co LTD-China. According to the instructions prostate tissue were cut and homogenized after adding Phosphate Buffer Saline (PBS) 9 times the prostate tissue weight. The supernatant collected after centrifuging at 3,000 rpm for 20 minutes. ELISA test done for prostate tissue concentration of the following biomarker: Tumor necrosis factor α, Prostate Specific Antigen (PSA) and Caspase 3.
Histological analysis and staining procedure: The prostate tissue cut and fixed in 10% formaldehyde. Prostate tissue then treated with alcohol 70%, 80 %, 90 % and absolute concentration of alcohol. The slide immersed in paraffin. Paraffin cleared using xylene. The slide cut at 4 κm using microtome. The slide then immersed in absolute, 90%, 80% and 70% alcohol in that order. The slide washed in water for 1 minute, stained with hematoxylin and eosin. The slide after that washed with water. The slide treated with 70%, 80%, 90% and absolute concentration of alcohol. At last Canada balsam added and the slide cleared using xylene. The slide read using light microscope.
Statistical analysis
The results analysis by using SPSS and value of each result expressed as a mean ± SD (Standard Deviation). The post hoc test was used, and p ≤ 0.05 was considered significant. P >0.05 was considered as no significant difference.
Results
The mean of PI, CPV and APV in all groups showed in Table 1.
| Rats group | Mean PI g/100 g ± S.D | Mean CPV mm3 ± S.D | Mean APV ml ± S.D |
|---|---|---|---|
| Normal | 0.208 ± 0.045 | 370.1 ± 100.3 | 330 ± 67.4 |
| Induction | 0.317 ± 0.053 | 997.9 ± 148.6 | 825 ± 139.9 |
| Conventional treatment | 0.244 ± 0.025 | 468 ± 123.4 | 385 ± 127 |
| Botulinum | 0.239 ± 0.056 | 472.6 ± 62 | 380 ± 94.8 |
| Nicotine | 0.338 ± 0.088 | 845 ± 177 | 845 ± 177 |
Note: SD: Standard Deviation; PI: Prostate Index; CPV: Calculated Prostate Volume; APV: Actual Prostate Volume; g: gram; mm3: cubic millimeter and ml: microliter.
Botulinum-Normal and conventional treatment groups showed a significant reduction in PI, CPV and APV when compared with induction and nicotine groups in post hoc ANOVA test.
Significant differences when (p ≤ 0.05).
Table 1: The means value of PI, CPV and APV of each group with standard deviation.
The mean of sTNFα, sPSA and sCASP3 in all groups showed in Table 2.
| Rats group | Mean sTNFα ng/ml ± S.D | Mean sPSA ng/ml ± S.D | Mean sCASP3 ± S.D |
|---|---|---|---|
| Normal | 78.2 ± 34.195 | 0.629 ± 0.126 | 3.004 ± 3.172 |
| Induction | 139.236 ± 34.691 | 1.023 ± 0.423 | 2.024 ± 0.505 |
| Conventional treatment | 99.545 ± 27.78 | 0.886 ± 0.188 | 3.639 ± 3.176 |
| Botulinum | 84.327 ± 28.063 | 0.685 ± 0.264 | 3.702 ± 2.547 |
| Nicotine | 157.836 ± 24.517 | 1.328 ± 0.356 | 1.882 ± 0.338 |
Note: SD: Standard Deviation; sTNFα: Serum TNFα level; sPSA: Serum PSA level and sCASP3: Serum caspase 3 level.
Botulinum-conventional treatment and normal groups showed a significant reduction in sTNFα induction and nicotine groups. Botulinum and normal group expressed a significant reduction in sPSA in comparison with induction and nicotine groups. Conventional treatment showed no significant reduction difference in sPSA in relation with induction and normal groups.
There is no significant difference in sCASP3 between all groups. Significant differences when (p value ≤ 0.05). Post hoc ANOVA test used to analyze the results.
Table 2: The mean and SD of sTNFα, sPSA and sCASP3 of each group.
The mean of pTNFα, pPSA and pCASP3 showed in Table 3.
| Rats group | Mean pTNFα ng/ml ± S.D | Mean pPSA ng/ml ± S.D | Mean pCASP3 ± S.D |
|---|---|---|---|
| Normal | 37.218 ± 6.415 | 0.315 ± 0.164 | 2.38 ± 1.627 |
| Induction | 84.054 ± 67.871 | 0.606 ± 0.394 | 2.151 ± 1.582 |
| Conventional treatment | 48.763 ± 27.662 | 0.411 ± 0.197 | 3.319 ± 3.504 |
| Botulinum | 41.272 ± 6.78 | 0.339 ± 0.213 | 10.843 ± 7.731 |
| Nicotine | 95.712 ± 47.056 | 0.65 ± 0.24 | 2.15 ± 1.444 |
Note: S.D: Standard Deviation; pTNFα: Prostate TNFα concentration; pPSA: Prostate PSA concentration and pCASP3: Prostate caspase 3 concentration.
Normal; conventional treatment and botulinum groups showed significant reduction in pTNFα when compared with induction group. Normal; botulinum groups showed significant reduction in pPSA when compared with induction group. Conventional treatment group showed no significant difference in pPSA with all other groups. Botulinum group increase pCASP3 significantly when compared with all other groups. Significant when p ≤ 0;05.
Table 3: The mean and SD of pTNFα, pPSA and pCASP3 of each group.
The degree of Prostate Percentage of Inhibition (PPI) for botulinum, conventional treatment and nicotine groups were 71.5%, 66.7% and -19.5% respectively.
Histopathological examination
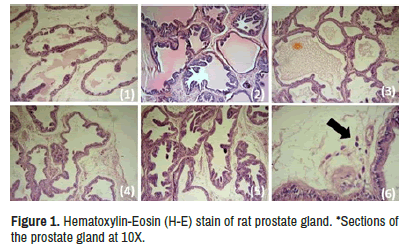
The section of the prostate tissue of the normal group showed unremarkable histopathological changes without sign of inflammation or hyperplasia. The induction group showed increase in proliferation of the glandular and stromal tissue with hyperplastic changes and increased number of the glands and cells. There are strong inflammatory reaction with numerous vascular dilation and congestion. The conventional treatment group showed mild hyperplastic changes with mild inflammatory reaction of the stroma. The investigated therapy group showed mild inflammatory reactions with mild hyperplasia and decrease prostate glands secretion with appearance of scattered apoptotic body. The nicotine group showed hyperplasia with strong stromal inflammatory reaction and focal hemorrhage with vascular congestion. Figure 1 showed those entire histological features.
a) Normal group b) Induction group c) Conventional treatment group d) Botulinum group e) Nicotine group *f) Section of the prostate gland at 40X of the investigated therapy group showed apoptotic body marked by black arrow.
Discussion
The results of this research suggest that use of BTXA for the treatment of BPH in rats showed a significant improvement in the measured parameters. It also clarify that there is a central role for nicotine in BPH progression and that effect of BTXA may be in large part due to inhibition of acetylcholine from stimulating prostate nicotinic receptor as nicotine administration reverse BTXA effects. It is also showed that BTXA decrease inflammatory cytokine TNFα, reduce prostate weight and volume and induce apoptosis. The study showed that continuous daily administration of conventional treatment with alfuzosin and dutasteride showed similar effect to single botulinum neurotoxin injection except that botulinum neurotoxin more powerful in reduce PSA and induce apoptosis.
When compared with induction group. Botulinum group showed a significant reduction in PI, CPV and APV which mean BTXA decreases weight and size of the prostate. This may be due to reduce number of cells in the prostate gland as a result of growth inhibition and apoptosis induction action. BXTA induce apoptosis in the prostate (26) by increasing pCASP3 as shown in Table 3 and the appearance of apoptotic body as shown in Figure 1a-1f. There is no significant difference in the sCASP3 between all groups. In apoptosis, the apoptotic body formed which consist of intact plasma membrane surround the cytoplasm and organelles, this apoptotic body mainly removed through phagocyte engulfment. The cell component not released into interstitial area surrounding the cells so, that’s explained why inflammation not occur in apoptosis [27]. These findings suggest sCASP3 is not reliable biomarker for testing actual prostate apoptotic activity.
BTXA growth inhibition action in the prostate may be due to interruption of α5 nAChR and Muscarinic receptor signals through inhibition of acetylcholine release from the parasympathetic nerve ending in the prostate gland. Inhibition of α5 nAchR in the prostate gland may cause decrease in Akt and ERK1/2 expression resulting in decrease prostate cells proliferation [28]. Akt shown to increase expression of AR receptor in androgen dependent prostate cancer [29]. It is also obvious that Akt inhibition cause increase the process of apoptosis as Akt cause inhibition of caspase 9 activation and at the premitochondrial level before release of cytochrome c [30]. Inhibition of ERK signaling in human prostate cancer cell result in down regulation of prostate cancer [31]. Activation of M3 receptor in prostate gland activate Ca+2/calmodulin signal lead to proliferation of prostate epithelial cells, increase prostate secretion and cause prostate smooth muscle contraction [32]. BXTA inhibit norepinephrine release leading to increase TGF-β and decrease expression of NF-κB, AR and 5α reductase [33,34].
In comparison with induction group, Botulinum group showed a significant reduction in sTNFα and pTNFα as shown in Tables 2 and 3 that may be an indicator that BTXA decrease systemic as well as local inflammation. TNFα is a cytokine have a central role in inflammation [35]. BTXA have anti-inflammatory effect in reducing mast cell degranulation in induced arthritis in rats [36]. Antagonizing the muscarinic receptor in rat’s leukocyte decreases T-cells and reduces chemoattraction to lymphocyte, monocyte and other neutrophil [37].
When compared with induction group, Botulinum group showed significant decrease in sPSA and pPSA in Tables 2 and 3. BTXA showed to decrease PSA in humans [38]. PSA is secreted from prostate gland [39]. This result is supported by evidence of decreased secretion in the glandular part of the prostate tissue histopathological examination in Figure 1a-1d.
Conventional treatment group significantly reduce PI, CPV and APV when compared with induction group. Results also showed that despite this effect conventional treatment group has not shown a significant increase in caspase 3 level in tissue or blood as shown in Tables 2 and 3. These finding suggest that conventional treatment group reduce size and weight of the prostate gland by a process other than apoptosis. α1 adrenergic antagonists shown to decrease proliferation by activity that include its action on blocking α1A receptors and by other mechanism that is not related to α1 A receptor like DNA damage as shown in [40]. Dutasteride is an 5α reductase inhibitor and researches showed that these type of drugs cause autophagy through down regulation of Insulin like Growth Factor 1 (IGF-1) in the stromal cells of the prostate glands [41]. Conventional treatment group showed a significant reduction in sTNFα and pTNFα when compared with induction group as shown Tables 2 and 3. Dutasteride treatment restored the expression of Estrogen Receptor β (ERβ) that was downregulated in the inflamed prostate. ERβ showed anti-inflammatory effect in human prostate [42]. Alfuzosin showed to reduce chronic prostatitis [43]. The conventional treatment group showed moderate reduction in both sPSA and pPSA as it showed no significant difference with both normal and induction group. The histopathological finding suggests that conventional treatment group not reduce prostate secretion as shown in Figure 1. Alfuzosin show no significant difference in PSA level between untreated patient group and patient group administered alfuzosin for at least one month [44]. It is well known that dutasteride reduce PSA level significantly after six months of treatment [45].
Nicotine group inverse the action BTXA on the prostate gland and showed significant increase in PI, CPV and APV when compared with botulinum, normal groups, on the other hand expressed no significant difference with induction group. Dr. Silva study suggests that the effect of this BTXA in the prostate related to its activity on the adrenergic not cholinergic system. The limitation of this study that it is just focused on study the muscarinic receptor implication through bethanicol which is a direct muscarinic receptor agonist but without give attention to prostate nicotinic receptor. It was thought that nicotine proliferative effect on prostate gland attributed to stimulation of nicotinic receptor located on the ganglia of neurons terminate on the prostate that lead to secretion of norepinephrine from nerve terminal [46]. For the BTXA used to block the release of norepinephrine from nerve terminal to just focus on effect of nicotine on the prostate nicotinic receptor as botulinum toxin block acetylcholine, norepinephrine and ATP release from nerve terminal [13]. α5nAChR activation increases proliferation and decreased phosphorylation of level of AKT and ERK1/2 [28]. Evidences in both in vivo and in vitro animal model studies suggest that treatment with nicotinic acetylcholine receptor blocker such as α-Bungarotoxin could reverse proliferation induced by nicotine [47]. Nicotine smoking is also known to inhibit aromatase enzyme responsible for conversion of androgen to estrogen so that is explain the reduction of estradiol 17β on female rats after chronic exposure to nicotine [48]. Nicotine increased testosterone level because rats that given aromatase inhibitor showed high testosterone level [49]. Nicotine reverse BTXA reducing inflammation effect as shown in Tables 2 and 3 that nicotine administration increase sTNFα and TNFα. Nicotine exposure from smoking increase inflammation [6]. Nicotine group showed significant decrease in pCASP3 as shown in Table 3. Nicotine suppresses apoptosis induced by cisplatin in lung cancer by causing bcl2 stability [50].
Conclusion
BTXA intraprostatic injection found to reduce hyperplasia by induction of apoptosis, reduce inflammation and decrease PSA that mean it eliminate the main key players in the pathogenesis of BPH. It also obvious from the results that BTXA as effective as the conventional treatment and have advantage of be more powerful in causing prostate growth inhibition, less side effects and a pharmaco-economic advantage of be single injection instead of daily medication. Nicotine administration reverses the effects of Botulinum neurotoxin type A that’s prove the central role of nicotinic receptor stimulation in the pathogenesis of BPH. Further studies need for studying the effects of repetitive doses of BTXA as well as to study the duration of action of BTAX in BPH treatment. The nicotinic receptor may be a new target for the future researches to develop new effective drugs to treat BPH.
References
- Ateyah, Mazin Abdulridha, Manal Khalid Abdulridha, and Munaim Jumaa Alkabee. "Saw Palmetto Therapy for Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia Assessment in Iraq." Medico Legal Update 21 (2021): 1448-1454.
- Li, Jing, Yanxin Tian, Shimeng Guo, and Haifeng Gu, et al. "Testosterone-Induced Benign Prostatic Hyperplasia Rat and Dog as Facile Models to Assess Drugs Targeting Lower Urinary Tract Symptoms." PLoS One 13 (2018): e0191469.
- Tiwari, Atul, NS Krishna, Kamna Nanda, and Anita Chugh. "Benign Prostatic Hyperplasia: An Insight into Current Investigational Medical Therapies." Expert Opin Investig Drugs 14 (2005): 1359-1372.
- Hong, Geum-Lan, Kyung-Hyun Kim, Shanika Karunasagara, and Ju-Young Jung. "Characterization of LC3 and p62 on Rat Prostate Lobe in Benign Prostate Hyperplasia Animal Model." Anat Biol Anthropol 33 (2020): 181-191.
- Kozlowski, Lynn T, Nicholas Y Mehta, Christine T Sweeney, and Stephen S Schwartz, et al. "Filter Ventilation and Nicotine Content of Tobacco in Cigarettes from Canada, the United Kingdom, and the United States." TobaccoControl 7 (1998): 369-375.
- Dwivedi, Shailendra, Apul Goel, Anil Mandhani, and Sanjay Khattri, et al. "Tobacco Exposure may Enhance Inflammation in Prostate Carcinoma Patients: An Explorative Study in North Indian Population." Toxicol Int 19 (2012): 310.
- Prueitt, Robyn L, Tiffany A Wallace, Sharon A Glynn, and Ming Yi, et al. "An Immune-Inflammation Gene Expression Signature in Prostate Tumors of Smokers." Cancer res 76 (2016): 1055-1065.
- Miller, J, and TH Tarter. "Combination Therapy with Dutasteride and Tamsulosin for the Treatment of Symptomatic Enlarged Prostate." Clin Interv Aging 4 (2009): 251.
- Jena, Ashish Kumar, Karan Vasisht, and Maninder Karan. "Therapeutic Management of Benign Prostatic Hyperplasia: From Synthetics to Naturals." Annu Res Rev Biol (2017): 1-34.
- Chen, Sheng. "Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments." Toxins4 (2012): 913-939.
- Satriyasa, Bagus Komang. "Botulinum Toxin (Botox) A for Reducing the Appearance of Facial Wrinkles: A Literature Review of Clinical Use and Pharmacological Aspect." Clin Cosmet Investig Dermatol 12 (2019): 223.
- Morales, Alberto Piamo, and Daisy Ferrer Marrero. "Effects of Botulinum Toxin on the Glandular Portion of the Prostate: Pathophysiologic Bases, Mechanism of Action, and Evidence." Revista Mexicana de Urología 80 (2020): 1-16.
- Ng, Lay Guat. "Botulinum Toxin and Benign Prostatic Hyperplasia." Asian J Urol 5 (2018): 33-36.
- Pirazzini, Marco, Ornella Rossetto, Roberto Eleopra, and Cesare Montecucco. "Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology." Pharmacol Rev 69 (2017): 200-235.
- Südhof, Thomas C. "Neurotransmitter Release: The Last Millisecond in the Life of a Synaptic Vesicle." Neuron 80 (2013): 675-690.
- McVary, Kevin T, Asim Razzaq, Chung Lee, and Mario F. Venegas, et al. "Growth of the Rat Prostate Gland is Facilitated by the Autonomic Nervous System." Biol Reprod 51 (1994): 99-107.
- Nguyen, Hoai Bac, Shin Young Lee, Soo Hyun Park, and Moo Yeol Lee, et al. "Relaxing Effect of Acetylcholine on Phenylephrine-Induced Contraction of Isolated Rabbit Prostate Strips is Mediated by Neuronal Nitric Oxide Synthase." Korean J Urol 54 (2013): 333-338.
- Zhao, Yue. "The Oncogenic Functions of Nicotinic Acetylcholine Receptors." J Oncol 20 (2016).
- Qi, Jinâ??Chun, Wenâ??Yong Xue, Yanâ??Ping Zhang, and Changâ??Bao Qu, et al. "Cholinergic α5 Nicotinic Receptor is Involved in the Proliferation and Invasion of Human Prostate Cancer Cells." Oncol Rep 43 (2020): 159-168.
- Obisike, UA, N Boisa, EO Nwachuku, and N. Nduka. "Antiproliferative Potentials of Zingiber officinale in Testosterone Induced Prostate Hyperplastic Albino Wister Rats." Int Res J Oncol (2020): 20-30.
- Obisike, UA, EO Nwachuku, N Boisa, and N Nduka. "Determination of Exogenous Testosterone Propionate Dose for Induction of Benign Prostatic Hyperplasia in Rat Model." Eur J Biomed Pharm Sci 6 (2019): 141-47.
- Becker, Adam M, Rick W Keck, Daniel S Murtagh, and Aaron B Becker, et al. "Prostatic Involution after Intraprostatic Injection of Cobra Toxin."J Urol 184 (2010): 2192-2196.
- Doggweiler, Ragi, Dirkâ?Henrik Zermann, Manabu Ishigooka, and Richard A Schmidt. "Botoxâ?Induced Prostatic Involution." Prostate 37 (1998): 44-50.
- Basiri, Mohsen, Majid Asadi-Shekaari, Masoud Ezzatabdipour, and Arash Sarv Azad, et al. "Immunohistochemistry Study on Androgen and Estrogen Receptors of Rat Seminal Vesicle Submitted to Simultaneous Alcohol-Nicotine Treatment." Cell J (Yakhteh) 18 (2016): 458.
- Aprikian, Saro, Murilo Luz, Fadi Brimo, and Eleonora Scarlata, et al. "Improving Ultrasound-Based Prostate Volume Estimation." BMC Urol 19 (2019): 1-8.
- King, Ashley, Adrienne Quirouet, and Courtenay K Moore. "Urologic Applications of Botulinum Toxin." Cleve Clin J Med 82 (2015): 456-464.
- Elmore, Susan. "Apoptosis: a Review of Programmed Cell Death." Toxicol Pathol 35 (2007): 495-516.
- Qi, Jinâ??Chun, Wenâ??Yong Xue, Yanâ??Ping Zhang, and Changâ??Bao Qu, et al. "Cholinergic α5 Nicotinic Receptor is Involved in the Proliferation and Invasion of Human Prostate Cancer Cells." Oncol Rep 43 (2020): 159-168.
- Ha, Susan, Rachel Ruoff, Nicole Kahoud, and Thomas F. Franke, et al. "Androgen Receptor Levels are Upregulated by Akt in Prostate Cancer." Endocr Relat Cancer 18 (2011): 245-255.
- Zhou, Honglin, Xin-Ming Li, Judy Meinkoth, and Randall N. Pittman. "Akt Regulates Cell Survival and Apoptosis at a Postmitochondrial Level." J cell biol 151 (2000): 483-494.
- Zhang, Jinsong, Jiansong Wang, Ting Luan, and Yigang Zuo, et al. "Deubiquitinase USP9X Regulates the Invasion of Prostate Cancer Cells by Regulating the ERK Pathway and Mitochondrial Dynamics."Oncol Rep 41 (2019): 3292-3304.
- Wang, Naitao, Bai-Jun Dong, Yizhou Quan, and Qianqian Chen, et al. "Regulation of Prostate Development and Benign Prostatic Hyperplasia by Autocrine Cholinergic Signaling through Maintaining the Epithelial Progenitor Cells in Proliferating Status."Stem Cell Rep 6 (2016): 668-678.
- Vickman, Renee E, Omar E Franco, Daniel C Moline, and Donald J Vander Griend, et al. "The Role of the Androgen Receptor in Prostate Development and Benign Prostatic Hyperplasia: A Review." Asian J Urol 7 (2020): 191-202.
- Anglin, IE, DT Glassman, and N. Kyprianou. "Induction of Prostate Apoptosis by α 1-adrenoceptor Antagonists: Mechanistic Significance of the Quinazoline Component." Prostate Cancer Prostatic Dis 5 (2002): 88-95.
- Al Hadithi, Hayfaa Salman, and Nadia Hameed Mohammed. "Significance of Serum Soluble Fas (sFas/CD95) and Tumor Necrosis Factor-Alpha (TNF-α) in Radiologically Diagnosed Patients with Uterine Leiomyoma." Al-Mustansiriyah J Pharm Sci17 (2018): 9-9.
- Choi, Jae Eun, Tyler Werbel, Zhenping Wang, and Chia Chi Wu, et al. "Botulinum Toxin Blocks Mast Cells and Prevents Rosacea like Inflammation."J Dermatol Sci 93 (2019): 58-64.
- Razani-Boroujerdi, Seddigheh, Muskaan Behl, Fletcher F Hahn, and Juan Carlos Pena-Philippides, et al. "Role of Muscarinic Receptors in the Regulation of Immune and Inflammatory Responses." J Neuroimmunol 194 (2008): 83-88.
- Rusnack, Susan R, and Steven A Kaplan. "The Use of Botulinum Toxin in Men with Benign Prostatic Hyperplasia." Rev Urol 7 (2005): 234.
- Ideo, Hiroko, Jumpei Kondo, Taisei Nomura, and Norio Nonomura, et al. "Study of Glycosylation of Prostate-Specific Antigen Secreted by Cancer Tissue-Originated Spheroids Reveals New Candidates for Prostate Cancer Detection." Sci Rep 10 (2020): 1-13.
- Batty, Mallory, Rachel Pugh, Ilampirai Rathinam, and Joshua Simmonds, et al. "The Role of α1-adrenoceptor Antagonists in the Treatment of Prostate and other Cancers." Int J Mol Sci 17 (2016): 1339.
- Yang, Boâ?Yu, Chenâ?Yi Jiang, Chenâ?Yun Dai, and Ruiâ?Zhe Zhao, et al. "5â?ARI Induces Autophagy of Prostate Epithelial Cells through Suppressing IGFâ?1 Expression in Prostate Fibroblasts." Cell Prolif 52 (2019): e12590.
- Mizoguchi, Shinsuke, Kenichi Mori, Toshitaka Shin, and Zhou Wang, et al. "Effects of Dutasteride in a Rat Model of Chemically Induced Prostatic Inflammation-Potential Role of Estrogen Receptor β." Prostate 80 (2020): 1413-1420.
- Kim, Kyu Shik, and Hong Sang Moon. "The Effect of Alpha-Blockers Monotherapy vs. Combination Antibiotic Therapy on Symptom Alleviation in Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome." UrogenitalTract Infection 13 (2018): 1-6.
- Jalo, Mohammed Ridha Joodi A. "Comparison of the Efficacy and Safety of Alfuzosin and Tamsulosin in Relieving Lower Urinary Tract Symptoms in Iraqi Patients with Benign Prostatic Hyperplasia (BPH): Observational Case Reference Study." Ann Trop Med Public Health 23 (2020): 231-434.
- Obinata, Daisuke, Shugo Suzuki, Yataro Yamanaka, and Tsuyoshi Yoshizawa, et al. "Low Reduction of Prostate Volume is a Significant Predictor of Prostate Cancer at Subsequent Biopsy in Patients with Dutasteride: A Retrospective Study." Andrologia 52 (2020): e13810.
- Silva, João, Rui Pinto, Tiago Carvallho, and Ana Coelho, et al. "Mechanisms of Prostate Atrophy after Glandular Botulinum Neurotoxin Type a Injection: An Experimental Study in the Rat." Eur Urol 56 (2009): 134-141.
- Wu, Chih-Hsiung, Chia-Hwa Lee, and Yuan-Soon Ho. "Nicotinic Acetylcholine Receptor-based Blockade: Applications of Molecular Targets for Cancer Therapy." Clin Cancer Res 17 (2011): 3533-3541.
- d’Adesky, Nathan D, Juan Pablo de Rivero Vaccari, Pallab Bhattacharya, and Marc Schatz, et al. "Nicotine Alters Estrogen Receptor-Beta-Regulated Inflammasome Activity and Exacerbates Ischemic Brain Damage in Female Rats."Int Mol Sci 19 (2018): 1330.
- Bajpai, Anurag, Peter J Simm, Stephen J McPherson, and Vincenzo C. Russo, et al. "Peripubertal Aromatase Inhibition in Male Rats has Adverse Long-Term Effects on Bone Strength and Growth and Induces Prostatic Hyperplasia." J Endocrinol 207 (2010): 27.
- Nishioka, T, LY Luo, L Shen, and H He, et al. "Nicotine Increases the Resistance of Lung Cancer Cells to Cisplatin through Enhancing Bcl-2 Stability." Br J Cancer 110 (2014): 1785-1792.
Citation: Ghazi Faisal, Majid, Ghazi Alabbassi M, and Raof IB. "Potential Effect of Botulinum Neurotoxin A in the Treatment of Induced Benign Prostatic Hyperplasia in Male Rats.” Clin Schizophr Relat Psychoses 15S(2021). Doi: 10.3371/CSRP.MFGM.080921
Copyright: © 2021 Majid FG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.