Research - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 4
New Hypotheses on the Role of Microglias in Ischemic Reperfusion Injury Secondary to Neurocysticercosis
Lourdes de Fatima Ibanez Valdes and Humberto Foyaca Sibat*Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 24-Jul-2023, Manuscript No. CSRP-23-107923; Editor assigned: 26-Jul-2023, Pre QC No. CSRP-23-107923 (PQ); Reviewed: 10-Aug-2023, QC No. CSRP-23-107923; Revised: 17-Aug-2023, Manuscript No. CSRP-23-107923 (R) ; Published: 24-Aug-2023, DOI: 10.3371/CSRP.DLHF.082423
Abstract
Background: Cysticercosis (Ct) is a preventable and eradicable zoonotic parasitic disease secondary to an infection caused by the larva form of pig tapeworm Taenia solium (Ts), which mainly seen in people living in developing countries. However, the number of carriers in developed countries increases gradually due to globalization and uncontrolled migration. In this study, we look for the role played by Microglia (Mg) in the pathogenesis of Intraparenchymal/Subarachnoid Neurocysticercosis (I-SNCC)/Ischemic-Reperfusion-Injury (IRI). After reviewing this issue, we formulate some hypotheses regarding to the role of Mg in this process and deliver some novel therapeutic approaches for I-SNCC/IRI.
Methods: We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like "Neurocysticercosis"; "pathogenesis of neurocysticercosis"; "comorbidity in NCC"; or "I-SNCC"; or "IRI;" or "NCC/IS;" or "Treatment of I-SNCC/IRI;" or “MPC;” or “ischemic stroke” or “subarachnoid neurocysticercosis” or “racemose neurocysticercosis”
Results: All selected manuscripts were peer-reviewed, and we did not find publications related to Mga/I-SNCC/IRI.
Comments and concluding remarks: We hypothesized the role played by Mg on the pathogenesis of I-SNCC on the role of Mg during the colloid/nodular stage of INCC and racemose NCC and an associated ischemic stroke base on the well-known benefits of Mg polarization.
Keywords
Cysticercosis • Neurocysticercosis • Microglia activation • Apoptosis • Pyroptosis • Necroptosis • PANoptosis • PANoptosome
Abbreviations
Aβ: Amyloid-β; AD: Alzheimer’s Disease; AIDS: Acquired Immune Deficiency Syndrome; ALS: Amyotrophic Lateral Sclerosis; AMPK: AMP-Activated Protein Kinase; Ap: Apoptosis; BBB: Blood-Brain Barrier; BDNF: Brain-Derived Neurotrophic Factor; c-FLIP: cellular FADD-Like Interleukin-1β Converting enzyme Inhibitory Protein; cIAP1: cellular Inhibitor of Apoptosis Protein 1; CNS: Central Nervous System; DRs: Activated Death Receptors; FADD: Fas-Associated Death Domain; fALS: familial Amyotrophic Lateral Sclerosis; FPI: Fluid Percussion Injury; GLT-1:Glutamate Transporter-1; HIV-1: Human Immunodeficiency Virus 1; ICH: Intracerebral Haemorrhage; IGF-1: Insulin-Like Growth Factor 1; IKK: IκB Kinase; iNOS: Inducible Nitric Oxide Synthase; InsP3: Inositol 1,4,5-tris Phosphate; IRI: Ischemia-Reperfusion Injury; GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor; MLKL: Mixed Lineage Kinase Domain-Like Protein; mTOR: mammalian Target Of Rapamycin; Nec-1: Necrostatin-1; NEMO: Nuclear factor-Kappa B Essential Modulator; NLRP3: NLR Family Pyrin Domain Containing 3; NLRC5: NOD-Like Receptor family CARD domain containing 5; NF-κB: Nuclear Factor kappa B; NMDAR:N-Methyl-D-Aspartic acid or N-Methyl-D-Aspartate Receptor; NOD: Nucleotide-binding Oligomerization Domain;NOX2:NADPH Oxidase 2; NLRP3:NOD-Like Receptor Protein 3; Nrf2: Nuclear factor erythroid 2-related factor; OPTN: Optineurin; PCD: Programmed Cell Death; PD: Parkinson’s Disease; PD-1: Programmed Cell Death-1; RIC: RIPK1-Inhibitory Compound; RIPK1: Receptor- Interacting Protein Kinase 1; RIPK3: Receptor-Interacting Protein Kinase 3; ROS: Reactive oxygen species; sALS: sporadic Amyotrophic Lateral Sclerosis; SN: Substantia Nigra; SOD1: Superoxide Dismutase 1; TAK1: Transforming growth factor β-Activated Kinase-1; TBI: Traumatic Brain Injury; TGF-β: Tumor Growth Factor-β; TICAM-1: TIR domain-Containing Adaptor Molecule 1; TLR: Toll-Like Receptor; TNF: Tumor Necrosis Factor; TNF-α: Tumour Necrosis Factor α; TNFR1: TNF Receptor 1; TRADD: TNFR-Associated Death Domain; TRIF: Toll/IL-1 Receptor domain-containing adaptor inducing IFN-β; TXA2:Thromboxane A2; VEGF: Vascular Endothelial Growth Factor; WNV: West Nile Virus; WT: Wild-Type; xCT: Cystine/Glutamate Antiporter.
Introduction
In the human host, adult tapeworms develop in the small intestine after ingesting cysticercus from unfreezing/undercooked contaminated pork meat. When eggs/proglottids are ingested by pigs or human’s beings then the oncospheres hatch in the gut mucosa and then penetrate the intestinal wall before disseminating to almost all the body excepting thin membranes, narrow cavities, hair, nail, cartilage, bone tissues or the adrenal gland. When the parasite invades the brain parenchymal, ventricular system, subarachnoid space, spinal cord, or optic nerves to form cysticerci it’s named neurocysticercosis.
Cysticercosis (Ct) is a preventable neglected zoonosis but eradicable parasitic disease secondary to a cestode infection by the larva form of the pork tapeworm Taenia solium (Ts), most often seen in people living in developing countries. Ct can infest any internal organ in humans and pigs, including the hair, nails, bone tissue, epidermis, cartilage, and the adrenal gland. When the cysticercus is in the cerebral parenchymal, intraventricular system, Subarachnoid Space (SAS), cerebellum, brainstem, optic nerve, or spinal cord, then it is best known as Neurocysticercosis (NCC), and the often-clinical manifestations are headache and epileptic seizures/epilepsy among other less frequent symptoms and signs [1-5]. Epileptic Seizure (ES) disorder and Epilepsy (Ep) are the most common symptom of Intraparenchymal NCC (INCC). We performed more than ten epidemiological investigations in rural areas around Mthatha (South Africa), confirming that NCC is the leading cause of secondary epilepsy. All ES and Ep respond very well to first line Antiseizure Medication (ASM) and Antiepileptic Drugs (AED) [6-15]. Likewise, lack of available AED due to COVID-19 restrictions or other reasons, including financial constrictions and poor compliance, leads to Status Epilepticus (SE) complications. Despite of, patients presenting refractory epilepsy secondary to NCC without other causes were never seen in our region in the past twenty-five years. The most used ASM are benzodiazepine, and the commonest AED are valproic acid and carbamazepine. Levetiracetam is used only in tertiary hospitals and is not available in our rural areas [16-19].
Humans are the final host for the adult tapeworm (taeniasis), whereas humans and pigs can be intermediate hosts carrying the cysticercus (larval form), a cyst, fluid-filled membrane vesicles with an eccentric scolex inside. When these cysts are ingested by undercooked contaminated pork meat, it goes to the gut, where scolex evaginates and it is attached to the intestinal mucosa wall by two crowns of hooks avoiding not to be expelled out of the intestine by peristaltic movement. At the gut, one or a maximum of two parasites matures into a 2-4 meters length tapeworm, constituted by a scolex, neck and 1200 proglottids. Gravid proglottids contain between 600 to 2000 fertile eggs, which pass into the soil after defecation on alternating days if the person is not constipated or has diarrhoea. In impoverished countries or economically poor regions inside of advantageous countries (like our area) where access to clean and safe water is not possible and predominates poor sanitation, poor food/personal hygiene, poor educational health, high level of poverty and free-roaming pigs with access to human faeces contaminated by Ts eggs, the incidence/prevalence of NCC is notably high. When the proglottids or eggs are ingested by contaminated water, food or via the faecal-oral route, the embryos are released from the egg into the gut and pass through the gut mucosa to the blood flow, which carries them to the target tissues, where they are transformed into cysticerci. Like human beings, pigs can ingest eggs and develop porcine cysticercosis [20-26]. Person-to-person transmission is relatively standard and explains how non-eaten pork peoples are infected and why the disease is present in developed countries without free-range pigs and even in places where the four stages of cysticercus in the brain parenchymal have been identified [12].
Recently, we reviewed several novel aspects of NCC associated with COVID-19 and Human Immunodeficiency Virus (HIV), the autoimmunity, meningeal lymphatic, glymphatic drainage, and the role of activated OLG/ OPC/NG2 in the pathogenesis of NCC clinical manifestations/complications/ outcome. As we documented before, activation of microglia and astrocytes is at the centre of NCC neuroinflammatory pathways either directly or indirectly due to their secretion of pro-inflammatory cytokines, upregulation of BBB disrupting proteinases and formation of an inhibitory glial scar [27].
In 2006, we commented on the clinical features and other aspects related to spinal cord NCC (SCNCC) [28], and recently we commented on the role played by pericytes on the pathogenesis of local neuroinflammation due to NCC, the healing process and outcome of the SCNCC [29].
Most glial cells support the CNS's primary functions, including maintaining homeostasis of Neurons (NC)/Glial Cells (GC). In addition, as is well-known that most Astrocytes (Ast), microglia, oligodendrocytes, schwann cells, and ependymal cells are capable of mitotic division. The Oligodendrocytes (Od) structure and role in NCC have been commented on recently by us [19], and comments on Astrocytes (Ac) in NCC will be published by our group near soon. Notwithstanding, Microglia (Mg) cell is the main aim of this study.
In 1860 Rudolf Virchow described the SNCC for the first time in history. He reported single and multicystic anatomical structures at the base of the brain mainly in subarachnoid cisterns. Two decades later Zenker informed to the medical community that it was causes by an atypical presentation of T. solium brain invasion and named it Cysticercus racemose due to its similarity to a bunch of grapes [30].
Recently, some authors have established the incubation period of SNCC is from 10 to 25 years and they described the stage 1 (SNCC) as cyst growth, proliferation and invasion into surrounding areas leading to space occupying lesion followed by remarkable immune response to cystic degeneration leading to phase 2 (arachnoiditis, hydrocephalus, IS, NI). Same author proposed and named phase 3 to the inactive presentation after successfully repeated several courses of 8-14 days therapy [31].
Mgs are relatively small space-supporting cells that contain a small cytoplasm with elongated nuclei of monocyte's source of mesoderm-derived and resident macrophages of the CNS involved in the development of brain tissue structure, phagocyting/removing waste metabolite, foreign/damage organisms, material, or cells. Mgs content intermediate filament vimentin/ nestin [32].
Twenty years back, we reported a case series presenting clinical manifestations of Is, dementia, active NCC and Binswanger's Disease. Two patients died due to thromboembolic complications. At that time, we hypothesized about the Taenia solium-microglial activation-coagulation disorder, glial disorders like Blood-Brain-Barrier (BBB) disturbances, Binswanger's disease and we proposed do not use anti-parasitic therapy for similar patients [2].
In 2018, we published one chapter related to SNCC and IS in epileptic patients based on cross sectional study made with three group of patients where we found that the odds of IS in NCC were 2.0 and 2.6 times greater in cases with INCC and SNCC, respectively. However, in patients with an associated HIV the risk of IRI increased three times more [14]. At that time, we did not review the role played by Mg in cases presenting I-NCC/IRI which is the main aid of this review.
Most of Mg's investigations considered the existence of two types of Mgs known as M1, which release toxic substances and inflammatory factors to inhibit pathogens, and M2, which exerts neuroprotective functions by promoting restoration of impaired immune responses and subsequent regeneration and nerve repair. In 1919, the Spanish neurologist Pio del Rio Hortega, also known as a "Father of Microglia", proposed the existence of Mg subtypes, but it was ignored until 2016 when his ideas were retaken. Nonetheless, it has been universally accepted that the Spanish scientist first described Mgs as a new type of GC produced in the yolk sac by Mp migration from the periphery into the CNS around 10.5 days of embryonic development. He also found that the cell body of some Mg was associated and interacted with the initial segment of the axons and called it "satellite" Mg, where the action potential start [33].
In 2020 and before COVID-19 pandemic, the World Health Organization (WHO) established that stroke is the second leading cause of death all over the world and its disabling nature and mortality rate increase the burden on individual families and the community and principal therapeutical approach are mechanical thrombectomy and intravenous thrombolysis when its indicated [34].
The principal goal of this study is to answer the following research questions. 1. What is the role of Mg in I-SNCC/IRI according to published information? And based on the information found to bring more light into the current gaps in our understanding of I-SNCC/IRI and elaborate new hypotheses to encourage other colleagues to perform better investigation of this matter.
Materials and Methods
A systematic search of EMBASE, Medline, Cochrane Library, Scopus, PsycINFO, Global Health, Health Management Information Consortium, and CINAHL was conducted to identify articles published between January 31st 2003 to January 31st 2023, followed by hand-searching of relevant journals.
Search strategy for this review
A systematic online search of manuscripts published from January 01st 2000 to January 31st 2023 was conducted using the selected databases. Two different searches were launched to cover the IS associated with I-SNCC infection and how Mg works on this pathological process. Therefore, we screened all publications related to the issues under the search terms "I-SNCC," "Mg activation" [MeSH], "Mg/I-SNCC" [MeSH], and Mg/I-NCC/IRI. Then, we identified all studies that were relevant to these issues. Additionally, we carefully checked the references/bibliography/ citations of each included manuscript. Later, we systematically searched: Global Health, CINAHL, Cochrane Library, Health Management Information Consortium, Web of Science (Clarivate Analytics), Embassy, Medline (Ovid), and Scopus (Elsevier). The predominant intention was to select the original research studies related to our search strategy. Following an accurate confident peer-review process, we selected those full-text written in Spanish, Portuguese, and English-language.
As before cited, all papers were retrieved using MeSH, and we only included aspects within the current work scope.
Inclusion and exclusion criteria
We also selected randomized controlled trials or quasi-experimental studies published in peer-reviewed journals. However, the studies were excluded if they evaluated interventions for other types of parasitic infections, other infections, or vascular problems because their aetiologies differ from Taenia solium infection. In addition, the review was also limited to studies involving adult patients and published in Spanish, Portuguese, or English. Documents reporting confident (classify as an absence of major biases), original data on IS associated with SNCC were eligible for inclusion.
Conference proceeding, textbooks and published abstracts were excluded because of lack of information on the methodology used. Case series with less than 20 participants, review papers without original data and letters to editor or editorial without original date were rejected.
Study selection
We performed the literature search and scanned all articles by title and abstract. LdeFIV and HFS independently screened articles for eligibility. It was followed by a discussion to establish consensus on which studies were included, mainly when there was ambiguity.
Quality appraisal
Four areas of study quality were assessed: selection bias, study design, health status, blinding process, reasons for dropouts or withdrawals, and data collection methods. In addition, LdeFIV independently carried out a methodological quality assessment and then verified by HFS.
Data extraction
A data extraction mechanism was developed to extract research data about the setting, study design, demographic profile of patients, methods, measurement tools and timing of assessments, and outcomes. The screening process was performed using an Excel® spreadsheet (Microsoft Corp., Redmond, WA). In addition, crucial information was extracted from either the primary article or an earlier published manuscript on the intervention for secondary data analysis studies. LdeFIV and HFS conducted the data extraction independently; the consensus was achieved through discussion among the authors.
Methods of analysis
Extracted data were initially synthesized using textual descriptions to determine the characteristics of the selected studies, and then they were grouped, clustered, and presented in tabular form.
Study and cohort selection
We selected prospective and retrospective case reports, cross-sectional studies, cohort studies, case-control studies, case series, reviews, controlled clinical trials, and meta-analyses releasing data on inclusion criteria.
Data collection process
The selected information is extracted from each manuscript with Microsoft Excel in a structured coding scheme. The data collected included IS/SNCC, IS/INCC, Mg/Mp/I-SNCC/IRI, clinical features, population size, age distribution, and the investigations used to confirm the final diagnosis when applicable. In cases where there was uncertainty regarding the interpretation of the selected data or how it could be used, we analysed the situation until we arrived at a mutual agreement. Some authors of selected primary studies were contacted by email when the article being reviewed contained unclear or missing information on the study design or their reported results.
Data synthesis and analysis
In some publications we used the Cochran's Q test to assess homogeneity across studies and the use of I2 index to summarize the total variability in proportion due to between-study variation was not included. Our study used aggregate data when necessary, following the guidelines of PRISMA.
Quality assessment of selected publications
Initially, all studies were screened for bias using the Jadad scoring system as usual and included only those with Jadad scores ≥ 4 [35] for further assessment.
Results
Literature search
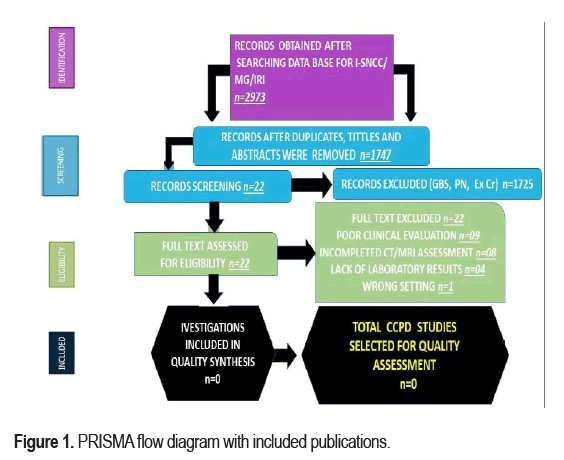
A total of 2973 manuscripts were of a sufficient quality to be selected for the first screening. Figure 1 which shows the number of articled selected in each database and included in level of bibliographic research and the main reasons for exclusions. During the screening phase nearly three-quarters of the manuscripts were excluded. An additional 1226 articles were excluded during the next phase, most of which (n = 0) did not show clinical evidence of I-SNCC/IS or did not confirm the diagnosis by neuroimaging. Finally, no articles were included for this research because they did not afford the role of Mg/Mp in I-SNCC/IS/IRI. From the beginning, all selected studies were peer-reviewed publications, but no one met all inclusion criteria on I-SNCC/ IS/IRI/Mg. Therefore, there has never been a systematic review of the role played by Mg in patients presenting IS associated to I-SNCC. A PRISMA flow chart for the literature searched is shown in Figure 1.
Study characteristics
Ethic committee did not consider this research work because it has not included bioethical implications. Most studies (79.1%) were published in the last four years. In South Africa, 49.44% of the population was HIV-positive, and 59.2% were female. Most investigations were conducted in the United States of America/Canada (42.1%), followed by Asia (39.5%), Africa (12.7) and European countries (5.7%). Most studies (77.3%) focused on people older than 18 years. The total of publications identified was n=2973; after duplicate removal, n=1747; after full text excluded, n=22; for quality synthesis, n=0; for quality assessment, n=0.
Discussion
Most studies combined case reports, cases-series, only two cross sectional studies, immunological analyses, and medical literature reviews. Some affected populations were probably not included in those reports because of the lack of proven diagnostic confirmation. In addition, due to scarce studies in children due to criteria diversity regarding to age group then many clinical features, demography, and immune response were not identified [1-9].
We grouped our series in four stages of NCC at the brain parenchymal, named as vesicular (stage 1) which is characterized by translucent wall with transparent fluid and a viable invaginated scolex with intact membrane, no-host immunological reaction, therefore, no Neuroinflammation (NI) around the cysts. Colloidal (stage 2): Here we see dying process of the parasite commonly before five years of entry [10-15]. Which is characterized by a cyst with a thick wall, turbid fluid, a degenerating scolex inducing a host inflammatory response. Here the intra-cystic fluid becomes turbid compared with the CSF density. The damaged membrane leaky liquid antigens damaging the BBB leading to vasogenic oedema surrounds the cyst [16-20]. In this stage, the neurological manifestations are more evident due to the direct/indirect effects of the released parasite's antigen. Granular/ nodular (stage 3): Decrease surrounding perilesional oedema, and the cyst begins to retract, but the enhancement persists also characterized by a cyst with a thicker wall, degenerated scolex, and little associated inflammatory response Calcified (stage 4): In some cases perilesional oedema can be present, all structural characteristic of the cyst disappears, and the remnant material is transformed into a coarse calcified nodule [21-29].
As we documented before, activation of Microglia (Mg)/Astrocytes (Ast) and Pericytes (Pc) are at the centre of NCC neuroinflammatory pathways either directly or indirectly due to the secretion of pro-inflammatory cytokines, upregulation of BBB disrupting proteinases and formation of an inhibitory glial scar [19,29,36,37].
Some authors have postulated that SNCC is the most lethal presentation of T solium infection into the brain as chronic NI, obstructive hydrocephalus, infectious vasculitis, stroke, cranial nerve disorders, and intracranial hypertension caused by several mechanisms including acute inflammatory reactions [14,31,38-41]. SNCC usually happened when cysticercus have the capacity of segmentation and development of interconnected new cyst (without scolex) and low or absent mechanical resistance to increase their volume up to become giant cysts [42]. However, other researchers hypothesized that the germinative cells are responsible to organize and maintain the expansive growth of the racemose larvae. They found proliferative cells within the bladder wall of SNCC and complete absence of those at the pericystic area in NCC at vesicular stage concluding that the growth of racemose larvae is due to abnormal cell proliferation [41]. Regarding to laboratory investigations some investigators confirmed the detection of cysticercus Ag in urine can be useful to identify asymptomatic cases of SNCC [43]. While many authors recommend the enzyme-linked immunoelectrotransfer blot as the best confirmatory serological test for SNCC with 70-98% of sensitivity and 100% specificity [14,44-46] and other investigators confirm the use of TsolR13-directed qPCR as the most sensitive (100%) test for detecting active SNCC in the CSF compartment [47].
Recently, it has been documented that in acute brain injury, CNS Mg are the first cells to respond and play a vital role in neural repair and regeneration but it can represent a double-edge phenomenon because Mg expression can also block the brain repair, augment the tissue damage has been report by other [48]. Therefore, we hypothesized that Mesenchymal Stem Cell (MSC)-derived Exosomes (Exos) which are nanoscale membrane vesicles also used as circulating biomarkers might be a future therapeutic candidate to treat NCC patients in critical condition due to their regenerative properties and immunomodulatory properties mainly in cases with NCC/ HIV/SARS-Co2/IS and other comorbidities leading to a very poor prognosis as we reported before [16-18] and based on that, we hypothesized about the role of Mg activation in NCC [2] and now we included the specific role of Mesenchymal Stem Cell-derived Exosomes (MSC-Exos) in regulating pro- inflammatory Mg in neuroinflammatory repair as it has been considered by other authors in cases of CNS injuries [48].
We hypothesized in previous articles about the mechanism of NI in the CNS very soon after the working relationship (ES) between the final/ intermediated host (humans) and the metacestode of the pork Taenia solium is broken by the abrupt release of T. solium antigens into the pericystic area triggering ischemic/inflammatory/autoimmune reaction together to oxidative stress, leading to NC excitotoxicity and PCD. We also considered that the surrounding NCC pericystic tissue damaged lead to increase acute inflammatory mediators plus proinflammatory cytokines mainly TNF-α, IL- 1β, IL-6, ROS, RNS, and ICAM-1, oxidative stress, and PANptosis including ferroptosis, causing disruption of BBB and pericystic vasogenic edema plus NI preceded by Mg activation.
Like Wan et al., we also considered that exosomes are extremely important vehicles for signalling among millions of cells and almost all human cells have the capacity of secret exosomes with obvious different regulatory functions due to their different structure and functions. Therefore, exosomes may have different regulatory effect in IRI/INCC compared with IRI/SNCC which explain their differences in clinical features, complications, PCD, and outcome. The role of Mg in NCC has been largely explained recently by us and we are not going to repeat here the same postulates due to publication page restriction. Last year, Wan and colleagues proved the close link between Mg polarization and exosomes and how microRNA (exosome inclusion eimRNA) regulate M1/M2 polarization. As before-cited miRNAs (endogenous hairpin-loop structured non-coding RNAs) among other functions, has the capacity to bind to messenger RNA to impede their translation and modulate gene activity [49].
As happened in other pathological conditions, in NCC the IS keep its hegemonic leadership as the commonest presentation of stroke accompanied by a series of ischemia, and ischemia/reperfusion events like oxidative stress, NI and dysfunctional BBB leading to disease progression. As we before cited the role played by activated Mg in M1 Mg will increase stroke-induced brain injury by promoting oxidative stress, NI, neuronal PANoptosis, glutamate excitotoxicity, and astrocyte differentiation, secreting IL-1β, IL-6, and TNF promoting NI and neurotoxic elements which aggravate brain lesion and M2 phenotype which secrete anti-inflammatory elements such as IL-4, IL-10, TGF-β plus neurotrophic factors promote myelin regeneration, BBB protection, neurogenesis, and neurite growth thus exerting a remarkable CNS protective function, and provide a better outcome as we commented in a previous publication [29,37].
Brief comments on the role of exosomes in I-SNCC/IRI
For communication with other cells, most eukaryotes and prokaryotes have the capacity to release Extracellular Vesicles (ExV) which are divided in many types predominantly in two types: Exosomes (EXS) and Ectosomes (ECS). EXS has a diameter between 50 nm to 1 um, are covered by two layers of phospholipid and it contains DNA, lipids, proteins, m/microRNA among other elements. It is known that EXS surge from endosomal/plasma membranes of antigen-presenting cells, reticulocytes, malignant cells, skeletal cell and many others and release their components into the recipient cell after fusing with them exerting a variety, diverse and powerful regulatory action [50]. We hypothesized that on top of the mediatory physiological effect, their participation in reproduction, immune response, cardiovascular, neurodegenerative, and malignant disorders EXS play an important role in the reported mechanism of PCD in NCC [19,20] by releasing EXS carrying the PCD-Ligand 1 at their membrane leading to upregulation of PD-L1 in the intracellular space of targeted cells. Notwithstanding, apart from protect the normal cells around the cysts EXS also interact with those pericystic cells as messenger of signalling intracellularly. Therefore, management of EXS in the therapy of IS/NCC should be applied considering they cross the BBB very easily, to be used for transportation of medications into the brain, and their capacity to transport some miRNA such as miR-124-3p triggering Mg polarization from resting Mg to anti-inflammatory M2 phenotype to inhibit NI and to promote tissues repair at the NCC/IRI area as proposed by other authors under different conditions [50,51].
Brief comments on Mg and NI and neurodegeneration associated to I-SNCC/IRI.
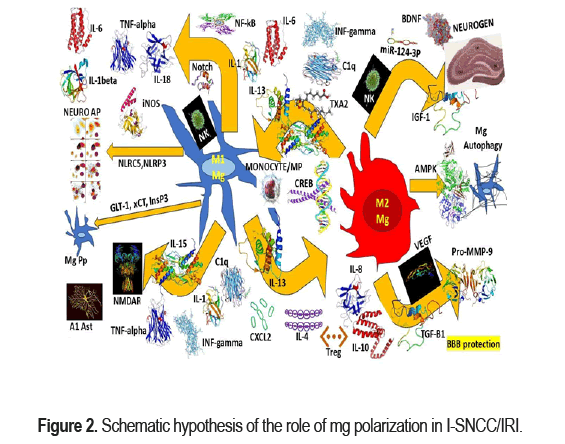
As we before-mentioned the Matrix Metalloproteinase (MMP), Reactive Oxygen Species (ROS), and pro-inflammatory cytokines released from activated Mg (M1) disrupt the BBB increasing local NI at the pericystic area during the colloid/nodular-fibrotic stage of NCC [19,20,29,37] and soon after the clearance system composed by MLV, GS, AQP4 and CA clean the damaged area [27]. we hypothesized that M2 Mg secrete anti-inflammatory cytokine IL-4/10 and neurotrophic factors (IFG-1) reducing the damaged ischemic/perfused area followed by promotion of neural repair. In patients presenting ischemic/reperfusion non-related with NCC a high expression of M2 markers (CD206) and M1 markers (MHCII) have been confirmed between 3 to 7 days after the stroke [52]. In Figure 2 we propose a graphical hypothesis related to the role of Mg polarization in patients presenting I-SNCC/IRI.
We hypothesized that after IRI, Mgs in the brain are activated, and peripheral monocytes infiltrate into the brain ischemic tissue. The MO phenotype then differentiate into M1 proinflammatory or M2 anti-inflammatory Mg, which play a beneficial or detrimental effect in the recovery of IRI. After activation, MPS secretes a variety of cytokines and interacts with other cells in the brain tissue, including astrocytes, oligodendrocytes, neutrophils. MPS can also phagocytose neurons to promote tissue repairing or aggravate tissue damage and support microvascular regeneration. On the other hand, we also hypothesized on the possible mechanism of brain- spleen-heart crosstalk. We believe that after IRI, there is an increase of the permeability of the BBB and the immune cells and inflammatory molecules in the brain reach the peripheral lymph nodes and spleen through the BBB to further regulate the occurrence of immune inflammation. The spleen releases NK cells and Treg cells into the brain to inhibit neuroinflammation while the monocytes promote NI. On top of that, the inflammatory reaction near the hypothalamus promotes the hypothalamic adrenaline axis to regulate the immune response of the spleen and heart damage. M1/M2 play many roles in IRI modulation. IL-13, IFN-γ, IL-15, IL-1, IL-6, TXA2 and C1q promote Mg M1 polarization or M2 Mg conversion to M1. TGF-β, P2X4, IL-4, IL-13, and Nrf2 promote Mg M2 conversion or M1 microglia conversion to M2. While activation of NF-κB and Notch in M1 microglia produce inflammatory factors and activation of NOX2 in M1 Mg leads ROS production. M1 microglia secrete ROS and TNF and thus induce neuronal Apoptosis. Increase expression of NLRP3 and NLRC5 in M1 microglia contribute to Mg scorching. The inhibition of InsP3 receptors facilitates the inhibition of glutamate release. M1 Mg secrete IL-1α, TNF-α, IFN-γ, C1q to promote the A1 phenotype of Ast. A1 phenotype astrocytes secrete GM- CSF and IL-15 to promote Mg M1 polarization. glial cell M1 polarization. IL-4, IL-13, TGF-β, P2X4, Nrf2 induce microglia M2 polarization. M2 Mg promote neurogenesis, remyelination and neurite outgrowth by secreting IGF-1, BDNF and miR-124-3p-containing exosomes. Increased AMPK or AKT/mTOR signaling promotes Mg autophagy, and enhanced autophagy is closely associated with Mg M2 polarization. M2 Mg promote BBB protection and rebuilding by secreting IL-8, IL-10, TGF-β1, VEGF and pro-MMP-9.
Based on reported ischemia-reperfusion studies we hypothesized that selecting CAMP-Responsive Element-Binding protein (CREB) in cases of I-SNCC, the M2-related genes expression can increase leading to nerve tissue repairing as was proved by Xia et al. [53] but under different circumstances Regarding to the Neuronal Regeneration (NRG) it is known that, the beneficial effect of Mg polarization into M2 phenotype as shown in Figure 2. We hypothesized that in NCC M2 which is mainly activated by IL-4 and able to release Vascular Endothelial Growth Factor (VEGF), Brain-Derived Neurotrophic Factor (BDNF), and Insulin-like Growth Factor-1 (IGF-1), they promote nerve repairs plus recovery/regeneration of the tissue damaged at the perilesional colloid/nodular/fibrotic NCC/IS. We also believe that BDNF in patients with IS/I-SNCC has the capacity to interact with TrkB receptors (activated by OLG) to increase OLG differentiation, and production of new myelin thickness layers as has been proved by other authors in cases with demyelination injury due other causes [54]. Recently we published some hypotheses on role of OLG/OPC in NCC which are available online for consultation [19].
On the other hand, Zabala and collaborators reported that myelin regeneration can be follow to administration of some anti-parasitic drugs like ivermectin by enhancing the P2X4R signalling pathway promoting resting Mg into M2 phenotype [55]. Simultaneously, Huang and colleagues found the neuritic outgrowth covered by new myelin layers can be promoted by secreted miR-124-3P (EXS/M2) and we hypothesized that increased secretion of M2 is one of the ideal future therapeutic approaches for complete recovering of the perilesional I-SNCC/IS [56] as shown in Figure 2.
As we before-mentioned, to collect an adequate number of cases and accurate research capacity to perform a well-designed clinical trial will be an extremely difficult task considering the proper limitations of developing countries and the low prevalence of I-SNCC in advantaged countries.
However, we recommended to keep in mind these therapeutic possibilities and to include Minocycline which is a semi-synthetic tetracycline antibiotic (second-generation) used to treat various bacterial infection, infectious diseases spread by lice, ticks, mites, and other non- infectious disease and has the capacity to attenuate the inhibitory function of neurotoxic M1 Mg on neurogenesis in Dorsal hippocampus during the oxidative stress according to the results published by Basset et al. [57] as shown in Figure 2.
Brief comments on the role Mg in I-SNCC/IRI Glutamate (GL) Excitotoxicity (ex)
The PCD in NCC were discussed recently by us [19,20]. Now we are to comment on the GLex mechanism for PCD in I-SNCC. It is worldwide accepted that ischemia/reperfusion/hypoxia affect the ATP synthesis leading to dysfunctional Na+/K+ pump depolarization of the cell membrane with the consequent Ca+ channel hyper expression and dysfunctional glutamate reuptake/N-Methy-D-Aspartate (NMDA) receptors/Ca+ overload/ peripheral neuronal death. We hypothesized that during the colloid/nodular/ fibrotic stage of I-SNCC/IS soon after Mg activation then GL is largely released from the Perspective of Glial Cells via xCT transport system and Glutamate Transporter 1 (GLT-1) has been proposed by other authors under other types of ischemia-reperfusion conditions [58]. Notwithstanding, we also postulate that in cases of I-SNCC/IS, Post-Synaptic Density 93 (PSD-93) binds to and specific chemokine (CX3 chemokine ligand 1) and mediating ischemia-induced glutamate excitotoxicity Mg is activated, as has been proposed for other cases of IS [58].
Brief comments on I-SNCC/IRI/Mga therapeutical approach
According to our data base, patients presenting SNCC have more chance to develop IS compared with patients with INCC and if they also have an associated HIV infection this frequency increase up to seven times more [14] and most of them remain chronically disable due to the ES/Ep, ischemic/reperfusion lesions, hydrocephalus (included those surgically treated), and other impaired tissue activities. Therefore, to apply novel therapeutical approaching to get better prognosis are mandatory. In this regard, we hypothesized that MSCs can help those patients based on their capacity to release many paracrine cytokines which might modify the immune microenvironment completely due to biosafety, nanometer size, facilities to cross the BBB, lack of immune response/immunomodulatory effect, potent short-term neuroprotective Mg effects, long-term neuroregenerative function, targeted delivery and being a remarkable carrier of drugs delivery to the CNS [48]. Based on the published results and our hypothetic postulates, allogenic MSC-Exos has gained an advantaged position and deserve to be included in the therapeutic management of IS/I-SNCC.
Taken into consideration the published results from administration of metformin and azithromycin in stroke models and their proven capacity to activate Mg leading neuroprotective phenotypes, we also considered these drugs should be included in the therapeutical arsenal for IS/I-SNCC as it has been proposed by other authors for other conditions [59].
As we before cited the Mg polarization to M2 leads anti-inflammatory effects included the role played by Notch-related signalling pathways and TLR (TLR3/IRF3) expression signalling pathway by plyinosinic-polycytidylic acid which has a proved therapeutic effect against brain ischemia/ reperfusion lesions, and furthermore inhibits TLR4/NF-κB signalling pathway reducing the vasogenic oedema and providing a better outcome which was first proved in experimental animal by Wang and collaborators [60].
We believe that controlling the signalling pathway of GL release Mg, modulating neuronal Ca+ metabolism/overload, inhibiting the ER inositol 1,4,5-trisphosphate receptor (vitamin C)-GL release and modulating G protein-coupled receptor 30 in patients presenting I-SNCC/IS, GLex is reduced, and neuroprotection is promoted as other authors proposed for different types of IS/aetiology [61]. Unfortunately, we should not sit down and wait for the conclusions/recommendation emerging from a large well- designed clinical trial because this process is an uncommon one and collect an adequate amount of data will take many years. In the same way, we included induced Pluripotent Stem Cell (iPSC)-derived MSCs (iMSCs), pioglitazone and rosiglitazone based on their capacity to regenerate the nervous system, reduce the MAPKs/NF-κB activity, reducing the ischemic/reperfusion area and the surrounding vasogenic oedema with neuroprotective effect [62]. We hypothesized that the first one is the best cell source for autologous cell therapy with remarkable regenerative effect for cases presenting IS/I-SNCC.
Recently, we published some hypotheses on the role of pericytes in NCC particularly in the mechanism of angiogenesis [29]. Now, we hypothesized on the role to be played by iMSC-Exos in IS/I-SNCC based on published reports from animal investigations [63] and capacity to inhibit the inflammatory proteins expression and to reduce the infarct area.
Unfortunately, one difference between prescribing metformin/ azithromycin and MSC-Exos is related with the previous pharmacological results obtained with the first one in other commonest clinical condition compared with MSC-Exos which needs further clinical trials to determine its effectiveness, sensitivity of drug administration, biological distribution and pharmacokinetics following bioethical principles apart from some exceptional conditions.
The role of EXS in I-SNCC promoting Mg polarization by releasing has-miR-124-3p and miR-30d-5p reducing the ischemic/reperfusion brain damage caused by NI was commented before. We like to highlight the importance of miR223-3p within EXS and its capacity to reduce I-R area and neuronal Ap by inhibition the NI response caused by M1 phenotype Mg as have been postulated by Liu et al. [64].
Nevertheless, same authors established that endavarone/melatonin/NGF with encapsulated EXS promote Mg polarization (M2) inhibiting NI as well [65]. Based on this evidence, we hypothesized that the application of EXS targeting Mg polarization and promoting M2 phenotype expressions should be included in the therapeutic arsenal of I-SNCC/IS for improving the outcome of the neurovascular complication of I-SNCC maintaining/restoring brain homeostasis as has been recommended by Liu et al. in patients presenting traumatic spinal cord injury by shifting M1/M2 polarization using EXS-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cell repairing [65] as shown in Figure 2.
For patients presenting I-SNCC/IS beyond the thrombolytic window, another recommended drug to be included in the treatment might be the vasoactive alkaloid vinpocetine due to its neuroprotective effects, increase blood flow and decrease resistance leading to an increased CNS perfusion (https://www.drugs. com/npp/vinpocetine.html.; https://www.drugs.com/npc/vinpocetine.html.) and based on other reports proving its capacity for promoting M2 polarization, enhancing autophagia and reducing neuronal damage [66]. On top of that, the capacity of EXS to cross the BBB makes them an ideal way of transportation for CNS drug delivery by implanting molecules on their lipid membrane with targeting properties.
Surgical approaches have been implemented in cases with SNCC, considering that the cysts can move freely through the SAS with unremarkable adherences to the NVS therefore to be removed surgically can be done easily with a gentle traction or by proper saline irrigation [67], while other authors highly stated that, the benefit of high doses of steroids, methotrexate and etanercept in patients with SNCC [68]. Based on the recent findings published by Kim et al. we speculated that in cases presenting I-SNCC/IS, reducing the Ifi2712a activity can reduce NI and cerebral IR area. These investigators proved that targeting Ifi2712a expression/function will alleviate CNS injury and NI after IS [69,70]. Other authors also proved that miRNAs have regulatory effects on Mg-associated inflammation and concluded that it can be confident therapeutic targets to improve IS outcomes and modulate NI [71]. While some immunomodulators interfering with miRNA can regulate Mg NI [72] and should be added to the list of therapeutic measurements including miRNA inhibitors.
Based on the comprehensive review of the medical literature, we can highlight the role of blood single-cell transcriptomics in cases presenting IRI as proposed by García-Bonilla and collaborators [73]. On other hand, we hypothesized that vitamin D deficiency or inactivation of vitamin D receptor (Mg/Mp) can cause poor prognosis of I-SNCC/IS by increasing secretion of TNF-α and IFN-γ which enhance CXCL10 from infiltration of peripheral T cells, BBB damaged and dysfunctional EC as has been documented by Pan Cui and collaborators [74]. Therefore, to administrate a supplement dosage of vitamin D in patients with I-SNCC/IS, should be taken into consideration.
Recently, Niu et al. documented that the anti-ageing nano-vesicles, inducible pluripotent stem cell-derived small extracellular vesicles (iPSC- sEVs) improve NI and promote polarization from M1/Mg to M2/Mg [75]. Therefore, we hypothesized, that iPSC-sEVs could alleviated Mg senescence in I-SNCC/IS providing neuroprotection and tissue repair.
A few months back, Wang et al. proved that in IR the activity of P2ry12 in Mg diminish remarkably and Mg migration toward BV is affected [76]; therefore, we hypothesized that the administration of P2ry12 inhibitors might improve the outcome of patients presenting I-SNCC/IRI.
Based on the scientific results published by Li et al. regarding the protective effect of Oleoylethanolamide (OEA) on Peroxisome Proliferator-Activated Receptor Alpha (PPARα)-M2 Mg polarization following IS [77], we hypothesize that OEA can be a potential new therapeutic drug for I-SNCC/ IS.
The before-cited information is summarized below in Table 1
| Approach | Mechanism | Results | Reference |
|---|---|---|---|
| Minocycline (Bacteriostatic antibiotic) | Activate M2 Mg/Mp around and within the peri-IRI areas, significantly decrease TNF-α and IL-1β levels, and increase TGF- β, IL-10 levels. | 78-(Gut-Brain Axis) | |
| Laquinimod | Activation of AhR | Reduces the inflammatory response and exerts neuroprotection after IS by inhibiting MPP-induced NLRP3 activation, reducing myelin loss and increasing protective natriuretic factors. | 79,80-(Gut-Brain Axis) |
| Lactobacillus reuteri | Activation of AhR | Function as an AhR ligand-fermenting probiotic | 81-(Gut-Brain Axis) |
| Etanercept | Peripheral administration of etanercept significantly improved motor function, and provide significant benefits for the chronic post-stroke management of pain in chronic stroke patients. | 82-(Gut-Brain Axis) | |
| Fingolimod | Targeting the crosstalk between the CNS and the peripheral immune system | Oral fingolimod is safe within 72 h, and limits secondary tissue damage after IS, reduces microvascular permeability, alleviates neurological deficits, and promotes recovery from baseline to 7 days. | 83-(Gut-Brain Axis) |
| Fingoli | Targeting the crosstalk between the CNS and the peripheral immune system | Whether the combination of the immunomodulators fingolimod and alteplase is safe and effective in reducing reperfusion injury in acute ischemic stroke patients treated within the first 4.5 h of symptom onset. | 84-(Gut-Brain Axis) |
| Fingolimod | Targeting the crosstalk between the CNS and the peripheral immune system | Target brain inflammation by skewing microglia toward M2 polarization after chronic cerebral hypoperfusion. | 85-(Gut-Brain Axis) |
| Bone Marrow Mesenchymal Stem Cell-Derived Exosomes | Modulating Microglia M1/M2 Phenotypes | Attenuate Cerebral Ischemia-Reperfusion Injury-Induced Neuroinflammation and Pyroptosis | 67 |
| Metformin | Activate Mg-leading neuroprotective phenotypes | 62 | |
| Azithromycin | Activate Mg-leading neuroprotective phenotypes | 62 | |
| Plyinosinic-polycytidylic acid | Inhibits TLR4/NF-κB signalling pathway reducing vasogenic oedema | Therapeutic effect against brain ischemia/reperfusion lesions | 63 |
| Pioglitazone and Rosiglitazone [Induced pluripotent stem cell (iPSC)-derived MSCs (iMSCs)] | Regenerate the nervous system, reduce the MAPKs/ NF-κB activity, reducing the IR area and the surrounding vasogenic oedema | Neuroprotective effect with a remarkable regenerative effect. | 65 |
| iMSC-Exos | Inhibit the expression of the inflammatory protein | Reduce the IR area. | 66 |
| miRNAs/miR223-3p | Inhibit the NI response caused by M1 phenotype Mg/ Regulatory effects on Mg-associated inflammation | Reduce IR area and neuronal Ap/Improve IS outcomes and modulate NI | 67,74,75 |
| Endavarone/Melatonin/N GF | Promote Mg polarization (M2) and inhibit NI | Improve the outcome of the NV complication | 67 |
| Vinpocetine (vasoactive alkaloid) | Increased blood flow and decreased resistance leading to an increased CNS perfusion. Promoting M2 polarization, enhancing autophagia | Neuroprotective effects reducing neuronal damage | 69 |
| Targeting Ifi2712a expression/function | Reducing the Ifi2712a activity | Reduce NI and IR area | 72,73 |
| Blood single-cell transcriptomics | No well known | Neuroprotective effect | 76 |
| Anti D deficiency or inactivation of vitamin D receptor | Decreasing the secretion of TNF-α and IFN-γ/enhance CXCL10 | To avoid BBB damaged and dysfunctional EC | 77 |
| inducible pluripotent stem cell-derived small extracellular vesicles (iPSC-sEVs) | Promote polarization from M1/Mg to M2/Mg | Reduce NI | 86 |
| P2ry12 inhibitors | To reduce activity of P2ry12 in Mg which diminishes remarkably Mg migration toward BV | Better outcome | 87 |
| Oleoylethanolamide (OEA) | Protective effect on peroxisome proliferator-activated receptor alpha (PPARα)-M2 Mg polarization | Neuroprotective effect | 88 |
| Carvedilol | Blockade adrenoreceptor | Inhibits the splenic response to stroke | 89 |
| Simvastatin | Attenuate s stroke-induced splenic atrophy. | Neuroprotective affect | 90 |
| Stem cell therapy | Unknown | Neuroprotective effect | 91 |
Brief comment on the role of mononuclear phagocyte system in I-SNCC/IRI
We hypothesized that the network/a collection of cell types also known as Mononuclear Phagocyte System (MPS) in cases of I-SNCC/IRI participate in the NI after its activation via chemotaxis around the lesion most probable from different subpopulations such as ant-inflammatory subset (CD14+CD16++) and the intermediate subset (CD14++CD16+) as has been proven by Ziqing and associates [86].
Recently, it has been established that Mononuclear Phagocyte System (MPS) plays a crucial beneficial role in patients presenting IS [86] and twenty years back, we hypothesized on the role of Mg activation in a series of patients presenting NCC/Binswanger disease [2]. Today, we know that monocytes/Mp/activated Mg located in the perilesional IR area share similar activities. While MPS is a leader of many neuroimmnological expressions such as phagocytosis/cytokine secretion, angiogenesis, immunoinflammatory response, potential biomarker, post stroke depression, recruitment/interaction of other auxiliary cells, and developing new therapeutic programs as has been proposed by other investigators [86].
Recently, Xu and colleagues have documented the role of Physical Exercise (PE) on the regenerative mechanism of the white matter injury after IRI by upregulation of TREM2 and Mg-derived factor for oligodendrocyte regeneration [87]. Based on the previous postulate we hypothesize that PE is a beneficial therapeutic approach to improve CNS functions/integrity in patients with I-SNCC/IS as well.
In 2021, we released some hypotheses on the role of microbiotas/ dysbiosis in haemorrhage stroke/COVID-19 [88]. Now we are going to deliver some comments about the role of immune cells travelling through the bidirectional communication pathway between the gut and the CNS (gut/brain axis-G/Ba) in cases presenting IS. Based on the results published by Zhou and collaborators [92], we hypothesized that as a swift response to the neuro insult created by I-SNCC/IS, the G/Ba conduct a disruption of the BBB, release pro-inflammatory elements, leads migration of inflammatory/ immune cells and DAMPs, dysbiosis, interact with Mg/Mp causing a decreased expression of IL-1β-6-17, and γδ Tregs while the expression of IL-10 and Tregs remarkable increase reducing all pro-inflammatory functions and promotes anti-inflammatory mechanisms. During this process, the participation of many G/Ba pathways such as AhR signaling pathways, LPS/ TLR4, TMAO/NLRP3, SCFA receptor, and HPA axis pathways serves to support this hypothesis and the proposal to administrate drugs involve in the management of peripheral immune system like a before-cited minocycline, etanercept, and fingolimod or activators of AhR like Lactobacillus reuteri or laquinimod, furthermore probiotics, Fecal Microbiota Transplantation (FMT) providing a better outcome of the patients. However, further investigations are needed to shed light on how the unbalance among Firmicutes and Bacteroides can modify the I-SNCC/IRI outcome by an ideal peripheral immune response and how the administration of supplementary probiotics/ FMT among other dietary measures and drugs supply can provide more benefits for those patients.
After a comprehensive analysis of the results published by Deng and collaborators [93], we hypothesized that NI caused by I-SNCC/IS characterized by M1/M2 Mg polarization increase the BBB permeability, disrupting the cardiovascular-related neural network which activate the ANS to regulate the NVS and cardiac function, plus modulation of the NK/Treg cells to entry into the CNS to avoid further NI, and the main components of the clearance system (MLV, GS, AQP4, and CA) [27]. On the other hand, neuro-cardiovascular disorder is caused by activated monocytes infiltration after I-SNCC/IS. Same authors established that hypothalamus (glutamic acid circuits) link/synchronize the ipsilateral Paraventricular Nucleus (PVN), the contralateral Supraoptic Nucleus (SON), and the RVLM to produce/regulate neural network expression/Hypothalamic-pituitary-Adrenal (HOA) axis (yo)/ catecholamine production/brain-spleen axis over expression/brain-heart crosstalk/cardiac monocyte hyper-infiltration/cardiovascular disorders (IRI, MI, HTA)/Mg polarization/TLR4 activation/Myeloid Differentiation primary response protein 88 (MyD88) pathway/TLR-associated Interferon (TRIF)/ Nuclear Factor-κB(NF-κB)/TNF-α and IL-6/AngII Type 1 Receptor (AT1R)/ EC-Mp/NI/neurogenic CVD/ high-mobility group protein box 1 (HMGB1)/PI- cytokines/Mg-P2Xr/“vicious cycle”/PCD (including splenocyte apoptosis). Nevertheless, we hypothesize that the management of TLR4 might improve clinical manifestations of neurogenic CVD.
Probable in cases of I-SNCC/IS decrease splenic volume after two/four days of the cerebrovascular insult can be observed as has been reported by Saand and colleagues under other conditions [94]. Based on experiments done in animals by same investigators, we hypothesized that administration of simvastatin in cases presenting I-SNCC/IRI may reduce spleen atrophy/ splenocyte apoptosis, IFN-β expression and frequency of lower respiratory tract infection. Until proven otherwise, the strong relationship between the spleen (the main peripheral immune organ) and I-SNCC/IRI raise a new concern to keep it in mind for future therapeutic strategies. Same author documented that some beta blocker drugs like carvedilol remarkable diminish the size of IRI caused by a large liberation of catecholamines on the splenic nerve after IRI increasing the immune activity of the spleen; therefore, we hypothesize that the same happen in I-SNCC/IRI.
Based on the previous search of the medical literature, we established the role of the neurovascular unit on the CNS homeostasis and BBB integrity [19] now other authors highly they implication in the pathophysiology of synucleinopathy and α-synuclein accumulation in patients presenting PD, LBD, MSA, and NI disorders which might include I-SNCC/IRI [95].
After deep analysis of the results published by Cao et al. [96] we also hypothesized that the anti-inflammatory properties of celastrol might serve to alleviate the NI in patients with I-SNCC/IRI by activation of Nrf2/HO-1 pathway and inhibiting NLRP3/caspase-1 pathway-mediated pyroptosis (NI-cytokines/Mg damage) and after a deep analysis of the publication of Shen et al. [97] we included in our hypotheses the role of Mg/Ast in scar formation/neurorepair/axon remodelling, neurogenesis, and Ag after I-SNCC/IRI. Taken into consideration the benefits of Apolipoprotein E (ApoE) as neuroprotective agent, its capacity to reverse Mg expression and supress cytokine production reported by Xue et al. [98] we hypothesized that ApoE might alleviate BBB dysfunction and NI following I-SNCC/IRI.
The neuroprotective function of caffeine has been reported by McLeod and collaborators [99]. Therefore, to include it in the list of potential neuroprotective substances able to provide additional benefits in cases presenting I-SNCC/IRI may be advisable.
Zeng et al. have documented the regulatory effect on Mgs of some natural plant components and proposed it as therapeutic element to keep the Mg balance during ischemic insult [100].
Conclusion
Therefore, we suggest it should be used in the management of I-SNCC/IRI as an anti-inflammatory procedure after its confirmation by an accurate clinical trial. Obviously, most of our hypotheses/proposals should be confirmed by well-designed clinical cohort studies for the collection of necessary data to create health and disease databases to develop standard operating protocol and clinical trials before to be included in the final management of I-SNCC/IRI. However, for those medications with no contraindication and free of side-effects its administration can be supported by the before-cited advantages. As far as we know, no previous review of this issue has been done.
Declaration
Consent for publication
We obtained the written informed consent for publication from our patient, including laboratory results. All information is fully available for any interested reader by request.
Ethical approval
The WSU/NMAH Ethical Committee did not request ethical approval for this study.
Competing interest
The authors declare that they performed this study without any commercial, financial, or otherwise relationships able to construe a potential conflict of interest.
Funding
All authors declare that they did not receive financial aid or collaboration that could have influenced the results reported in this paper.
Author’s contributions
Study concept and design: HFS and LFIV. Data collection from searched literature: HFS and LdeFIV. Analysis of the obtained data was done by LdeFIV/HFS plus the first and final draft of this paper. Manuscript was revised by HFS and LFIV and it was supervised by HFS. Manuscript writing process: HFS and LFIV. All authors have approved this version for publication.
Declaration of anonymity
All authors certified that they did not mention names, initials, and other identity issues of this patient. Therefore, a complete anonymity is guaranteed.
Availability of data and material
All data supporting this study are available on reasonable request from the corresponding author.
Acknowledgement
Special thanks to Dr Sibi Joseph for his participation in the management of our patients, Dr Khulile Moetketsi: Head of Department of Internal Medicine and Therapeutics of Nelson Mandela Academic Hospital. Professor Thozama Dubula: The Dean of the Faculty of Health Sciences of Walter Sisulu University.
References
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Pseudoseizures and Epilepsy in Neurocysticercosis." Rev Electron Biomed 2 (2003): 79-87.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Vascular Dementia Type Binswanger's Disease in Patients with Active Neurocysticercosis." Rev Electron Biomed 1 (2003): 32-42.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Insular Neurocysticercosis: Our Finding and Review of the Medical Literature." Intern J Neurol 5 (2006): 31-35.
- Foyaca-Sibat, Humberto, Linda D Cowan, Helene Carabin, and Irene Targonska, et al. "Accuracy of Serological Testing for the Diagnosis of Prevalent Neurocysticercosis in Outpatients with Epilepsy, Eastern Cape Province, South Africa." PLoS Negl Trop Dis 3 (2009): e562.
- Foyaca-Sibat, Humberto, LdeF Ibanez Valdes, and J Moré-Rodríguez. "Parasitic Zoonoses of the Brain: Another Challenger." Intern J Neurol 12 (2010): 9-14.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Treatment of Epilepsy Secondary to Neurocysticercosis". Intech Open (2011).
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Clinical Features of Epilepsy Secondary to Neurocysticercosis at the Insular Lobe." Intech Open 2011.
- Foyaca-Sibat, Humberto. "Epilepsy Secondary to Parasitic Zoonoses of the Brain." Intech Open 2011.
- Foyaca-Sibat, Humberto, M Salazar-Campos, and LdeF Ibanez Valdes. "Cysticercosis of the Extraocular Muscles. Our Experience and Review of the Medical Literature." Intern J Neurol 14 (2012).
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Introduction to Cysticercosis and Its Historical Background." Intech Open (2013).
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "What is a Low Frequency of the Disseminated Cysticercosis Suggests that Neurocysticercosis is Going to Disappear?." Intech Open 2013.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Uncommon Clinical Manifestations of Cysticercosis." Intech Open 8 (2013): 199-233.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Psychogenic Nonepileptic Seizures in Patients Living with Neurocysticercosis." Intech Open (2018).
- Foyaca-Sibat, Humberto, and Lourdes de Fátima Ibañez-Valdés. "Subarachnoid Cysticercosis and Ischaemic Stroke in Epileptic Patients." Intech Open (2018): 153.
- Noormahomed, Emilia Virgínia, Noémia Nhancupe, Jerónimo Mufume, and Robert T Schooley, et al. "Neurocysticercosis in Epileptic Children: An Overlooked Condition in Mozambique, Challenges in Diagnosis, Management and Research Priorities." EC Microbiol 17 (2021): 49-56.
- Humberto Foyaca Sibat. "Racemose Neurocysticercosis Long COVID and Brainstem Dysfunction: A Case report and Systematic Review”. Clin Schizophr Relat Psychoses 15S (2021).
- Foyaca-Sibat, Humberto. "Neurocysticercosis, Epilepsy, COVID-19 and a Novel Hypothesis: Cases Series and Systematic Review." Clin Schizophr Relat Psychoses 15 (2021): 1-13.
- Foyaca-Sibat, Humberto. "People Living with HIV and Neurocysticercosis Presenting Covid-19: A Systematic Review and Crosstalk Proposals." Clin Schizophr Relat Psychoses 15 (2021): 1-9.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Novel Hypotheses on the Role of Oligodendrocytes in Neurocysticercosis. Comprehensive Review". Clin Schizophr Relat Psychoses 17 (2023)
- Foyaca-Sibat, Humberto. "Comorbidity of Neurocysticercosis, HIV, Cerebellar Atrophy and SARS-Cov-2: Case Report and Systematic Review." Clin Schizophr Relat Psychoses 15 (2021): 1-6.
- Del Rio-Romero, A H, H Foyaca-Sibat, L de F Ibanez-Valdes, and E Vega-Novoa. "Prevalence of Epilepsy and General Knowledge about Neurocysticercosis at Nkalukeni Village, South Africa." Intern J Neurol 3 (2005): 1-6.
- Del Rio-Romero, A H, H Foyaca-Sibat, L de F Ibanez-Valdes, and E Vega-Novoa. "Neuroepidemiological Survey for Epilepsy and Knowledge about Neurocysticercosis at Ngqwala Location, South Africa." Intern J Neurol 4 (2004): 1-6.
- Foyaca-Sibat, Humberto, A Del Rio-Romero, and L. Ibanez-Valdes. "Prevalence of Epilepsy and General Knowledge about Neurocysticercosis at Ngangelizwe Location, South Africa." Intern J Neurol 4 (2005): 23-37.
- Del Rio-Romero, A H, and H Foyaca-Sibat. "Epidemiological Survey about Socio-Economic Characteristic of Mpindweni Location, South Africa." Intern J Neurol 4 (2005): 18-26.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Refractory Epilepsy in Neurocysticercosis." Intern J Neurol 5 (2005): 1-6.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Insular Neurocysticercosis: Our Finding and Review of the Medical Literature." Intern J Neurol 5 (2006): 31-35.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Meningeal Lymphatic Vessels and Glymphatic System in Neurocysticercosis. A Systematic Review and Novel Hypotheses." Clin Schizophr Relat Psychoses 17 (2023): 1-11.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Co-morbidity of Spinal Cord Neurocysticercosis and Tuberculosis in a HIV-Positive Patient." Intern J Neurol 7 (2007): 5-10.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. " The Role of Pericytes inNeurocysticercosis: Comprehensive Review and Novel Hypotheses." Clin Schizophr Relat Psychoses 17 (2023): 1-12.
- Zenker, FA. "Ueber den Cysticercus racemosus des Gehirns." Cohen (1882): 119-140.
- Nash, Theodore E, and Elise M. O’Connell. "Subarachnoid Neurocysticercosis: Emerging Concepts and Treatment." Curr Opin Infect Dis 33 (2020): 339.
- Ludwig, Parker E, and Joe M Das. "Histology, Glial Cells." StatPearls (2022).
- Wang, Jie, Wenbin He, and Junlong Zhang. "A Richer and More Diverse Future for Microglia Phenotypes." Heliyon 9 (2023): e14713.
- Thiebaut, Audrey M, Maxime Gauberti, Carine Ali, and Sara Martinez De Lizarrondo, et al. "The Role of Plasminogen Activators in Stroke Treatment: Fibrinolysis and Beyond." Lancet Neurol 17 (2018): 1121-1132.
- Jadad, Alejandro R, Andrew Moore R, Dawn Carroll, and Crispin Jenkinson, et al. "Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary?." Control Clin Trials 17 (1996): 1-12.
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. "Introduction to Novel Motor Neuron Disease." Intech Open (2019).
- Foyaca-Sibat, Humberto, and LdeF Ibanez Valdes. Novel Hypotheses on the Role of Microglia and Panoptosis in Neurocysticercosis: A Comprehensive Review. Clin Schizophr Relat Psychoses 17 (2023)
- Bazan, Rodrigo, Pedro Tadao Hamamoto Filho, Gustavo José Luvizutto, and Hélio Rubens de Carvalho Nunes, et al. "Clinical Symptoms, Imaging Features and Cyst Distribution in the Cerebrospinal Fluid Compartments in Patients with Extraparenchymal Neurocysticercosis." PLoS Negl Trop Dis 10 (2016): e0005115.
- Nash, Theodore E, Elise M. O’Connell, Dima A Hammoud, and Lauren Wetzler, et al. "Natural History of Treated Subarachnoid Neurocysticercosis." Am J Trop Med Hyg 102 (2020): 78-89.
- Abanto, Jesus, Daniel Blanco, Herbert Saavedra, and Isidro Gonzales, et al. "Mortality in Parenchymal and Subarachnoid Neurocysticercosis." Am J Trop Med Hyg 105 (2021): 176-180.
- Orrego, Miguel A, Manuela R Verastegui, Carlos M Vasquez, and Uriel Koziol, et al. "Identification and Culture of Proliferative Cells in Abnormal Taenia Solium Larvae: Role in the Development of Racemose Neurocysticercosis." PLoS Negl Trop Dis 15 (2021): e0009303.
- Marcin Sierra, Mariana, Mariana Arroyo, May Cadena Torres, and Nancy Ramírez Cruz, et al. "Extraparenchymal Neurocysticercosis: Demographic, Clinicoradiological, and Inflammatory Features." PLoS Negl Trop Dis 11 (2017): e0005646.
- McCleery, Ellen, Samantha E Allen, Luz Maria Moyano, and Ricardo Gamboa, et al. "Population Screening for Urine Antigens to Detect Asymptomatic Subarachnoid Neurocysticercosis: A Pilot Study in Northern Peru." Am J Trop Med Hyg 103 (2020): 1125.
- Theodoros, Kelesidis. "Extraparenchymal Neurocysticercosis." Intech Open (2013).
- Parra-Cárdenas, Diana Maritza, María Teresa Vargas-Cuervo, Jorge Armando Montejo-Coy, and Carlos Mauricio Calderon-Vargas, et al. "Subarachnoid Racemose Neurocysticercosis with Cerebellar Involvement: An Old Friend in an Infrequent Location?." Rev Inst Med Trop Sao Paulo 63 (2021): e43.
- Mahale, Rohan R, Anish Mehta, and Srinivasa Rangasetty. "Extraparenchymal (Racemose) Neurocysticercosis and Its Multitude Manifestations: A Comprehensive Review." J Clin Neurol 11 (2015): 203-211.
- O’Connell, Elise M, Sarah Harrison, Eric Dahlstrom, and Theodore Nash, et al. "A Novel, Highly Sensitive Quantitative Polymerase Chain Reaction Assay for the Diagnosis of Subarachnoid and Ventricular Neurocysticercosis and for Assessing Responses to Treatment." Clin Infect Dis 70 (2020): 1875-1881.
- Liu, Yu-Yan, Yun Li, Lu Wang, and Yan Zhao, et al. "Mesenchymal Stem Cell-Derived Exosomes Regulate Microglia Phenotypes: A Promising Treatment for Acute Central Nervous System Injury." Neural Regen Res 18 (2023): 1657.
- Wan, Teng, Yunling Huang, Xiaoyu Gao, and Wanpeng Wu, et al. "Microglia Polarization: A Novel Target of Exosome for Stroke Treatment." Front Cell Dev Biol 10 (2022): 842320.
- Kalluri, Raghu, and Valerie S LeBleu. "The Biology, Function, and Biomedical Applications of Exosomes." Science 367 (2020): eaau6977.
- Khan, Haroon, Jia-Ji Pan, Yongfang Li, and Zhijun Zhang, et al. "Native and Bioengineered Exosomes for Ischemic Stroke Therapy." Front Cell Dev Biol 9 (2021): 619565.
- Mirza, Mehwish A, Yan Xu, Louise D McCullough, and Fudong Liu. "Role of IRF5-IRF4 Regulatory Axis in Microglial Polarization after Neonatal Stroke." Stroke 46 (2015): A20-A20.
- Xia, Cong-Yuan, Shuai Zhang, Yan Gao, and Zhen-Zhen Wang, et al. "Selective Modulation of Microglia Polarization to M2 Phenotype for Stroke Treatment." Int Immunopharmacol 25 (2015): 377-382.
- Fletcher, Jessica L, Rhiannon J Wood, Jacqueline Nguyen, and Eleanor ML Norman, et al. "Targeting Trkb with a Brain-Derived Neurotrophic Factor Mimetic Promotes Myelin Repair in the Brain." J Neurosci 38 (2018): 7088-7099.
- Zabala, Alazne, Nuria Vazquez-Villoldo, Björn Rissiek, and Jon Gejo, et al. "P2X4 Receptor Controls Microglia Activation and Favors Remyelination in Autoimmune Encephalitis." EMBO Mol Med 10 (2018): e8743.
- Huang, Shan, Xintong Ge, Jinwen Yu, and Zhaoli Han, et al. "Increased miRâ?124â?3p in Microglial Exosomes Following Traumatic Brain Injury Inhibits Neuronal Inflammation and Contributes to Neurite Outgrowth via their Transfer into Neurons." FASEB J 32 (2018): 512-528.
- Bassett, Ben, Selvaraj Subramaniyam, Yang Fan, and Seth Varney, et al. "Minocycline Alleviates Depression-Like Symptoms by Rescuing Decrease in Neurogenesis in Dorsal Hippocampus via Blocking Microglia Activation/Phagocytosis." Brain Behav Immun 91 (2021): 519-530.
- Belov Kirdajova, Denisa, Jan Kriska, Jana Tureckova, and Miroslava Anderova. "Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells." Front Cell Neurosci 14 (2020): 51.
[Crossref] [Google Scholar] [PubMed]
- Yeh, Jiann-Horng, Kuo-Ching Wang, Asuka Kaizaki, and Jonathan W Lee, et al."Pioglitazone Ameliorates Lipopolysaccharide-Induced Behavioral Impairment, Brain Inflammation, White Matter Injury and Mitochondrial Dysfunction in Neonatal Rats." Int J Mol Sci 22 (2021): 6306.
[Crossref] [Google Scholar] [PubMed]
- Wang, Peng-Fei, Huang Fang, Jing Chen, and Sen Lin, et al. "Polyinosinic-Polycytidylic Acid has Therapeutic Effects against Cerebral Ischemia/Reperfusion Injury through the Downregulation of TLR4 Signaling via TLR3." J Immunol 192 (2014): 4783-4794.
[Crossref] [Google Scholar] [PubMed]
- Wan, Teng, Yunling Huang, Xiaoyu Gao, and Wanpeng Wu, et al. "Microglia Polarization: A Novel Target of Exosome for Stroke Treatment." Front Cell Dev Biol 10 (2022): 842320.
[Crossref] [Google Scholar] [PubMed]
- Lee, Won Hee, Wen-Yi Chen, Ning-Yi Shao, and Dan Xiao, et al. "Comparison Of Non-Coding RNAs in Exosomes and Functional Efficacy of Human Embryonic Stem Cell-Versus Induced Pluripotent Stem Cell-Derived Cardiomyocytes." Stem Cells 35 (2017): 2138-2149.
[Crossref] [Google Scholar] [PubMed]
- Chen, Kuan-Hung, Kun-Chen Lin, Christopher Glenn Wallace, and Yi-Chen Li, et al. "Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cell Therapy Effectively Reduced Brain Infarct Volume and Preserved Neurological Function in Rat after Acute Intracranial Hemorrhage." Am J Transl Res 11 (2019): 6232-6248.
[Google Scholar] [PubMed]
- Liu, Xiaoli, Meimei Zhang, Haining Liu, and Rui Zhu, et al. "Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate Cerebral Ischemia-Reperfusion Injury-Induced Neuroinflammation and Pyroptosis by Modulating Microglia M1/M2 Phenotypes." Exp Neurol 341 (2021): 113700.
[Crossref] [Google Scholar] [PubMed]
- Liu, Wei, Yuluo Rong, Jiaxing Wang, and Zheng Zhou, et al. "Exosome-Shuttled miR-216a-5p from Hypoxic Preconditioned Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Shifting Microglial M1/M2 Polarization." J Neuroinflammation 17 (2020): 47.
[Crossref] [Google Scholar] [PubMed]
- Zhang, Qida, Zhenxian Chen, Zhifeng Zhang, and Zhongmin Jin, et al. "Leveraging Subject-Specific Musculoskeletal Modeling to Assess Effect of Anterior Cruciate Ligament Retaining Total Knee Arthroplasty during Walking Gait." Proc Inst Mech Eng H 234 (2020): 1445-1456.
[Crossref] [Google Scholar] [PubMed]
- Lines, William W, Juan Luis Gómez-Amador, Hector H García, and Jorge E Medina, et al. "Endoscopic Endonasal Surgery for Massive Subarachnoid Neurocysticercosis: Illustrative Case." J Neurosurg Case Lessons 2 (2021): CASE21366.
[Crossref] [Google Scholar] [PubMed]
- White Jr, A Clinton, and Agnes Fleury. "Optimal Treatment for Subarachnoid Neurocysticercosis: Closer, but not there Yet." Am J Trop Med Hyg 102 (2020): 1-2.
[Crossref] [Google Scholar] [PubMed]
- Kim, Eunhee, and Sunghee Cho. "Microglia and Monocyte-Derived Macrophages in Stroke." Neurotherapeutics 13 (2016): 702-718.
[Crossref] [Google Scholar] [PubMed]
- Kim, Gab Seok, Elisabeth Harmon, Manuel Gutierrez, and Jessica Stephenson, et al. "Single-Cell Analysis Identifies Ifi27l2a as a Novel Gene Regulator of Microglial Inflammation in the Context of Aging and Stroke." Res Sq (2023): rs-3.rs-2557290.
[Crossref] [Google Scholar] [PubMed]
- Lian, Lu, Yunsha Zhang, Lu Liu, and Liji Yang, et al. "Neuroinflammation in Ischemic Stroke: Focus On MicroRNA-Mediated Polarization of Microglia." Front Mol Neurosci 13 (2021): 612439.
[Crossref] [Google Scholar] [PubMed]
- Martinez, Bridget, and Philip V Peplow. "Immunomodulators and microRNAs as Neurorestorative Therapy for Ischemic Stroke." Neural Regen Res 12 (2017): 865-874.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Bonilla, Lidia, Ziasmin Shahanoor, Rose Sciortino, and Omina Nazarzoda, et al. "Brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke." bioRxiv (2023): 2023-03.31.535150.
[Crossref] [Google Scholar] [PubMed]
- Cui, Pan, Wanting Lu, Junjie Wang, and Fei Wang, et al. "Microglia/Macrophages Require Vitamin D Signaling to Restrain Neuroinflammation and Brain Injury in a Murine Ischemic Stroke Model." J Neuroinflammation 20 (2023): 63.
[Crossref] [Google Scholar] [PubMed]
- Niu, Xinyu, Yuguo Xia, Lei Luo, and Yu Chen, et al. "iPSC-sEVs Alleviate Microglia Senescence to Protect against Ischemic Stroke in Aged Mice." Mater Today Bio 19 (2023): 100600.
- Wang, Chenglong, Li Peng, Yuan Wang, and Ying Xue, et al. "Integrative Analysis of Single-Cell and Bulk Sequencing Data Depicting the Expression and Function of P2ry12 in Microglia Post Ischemia–Reperfusion Injury." Int J Mol Sci 24 (2023): 6772.
[Crossref] [Google Scholar] [PubMed]
- Li, Ying, Yanan Zhang, Qing Wang, and Chuang Wu, et al. "Oleoylethanolamide Protects against Acute Ischemic Stroke by Promoting Pparα-Mediated Microglia/Macrophage M2 Polarization." Pharmaceuticals (Basel) 16 (2023): 621.
[Crossref] [Google Scholar] [PubMed]
- Yang, Yirong, Victor M Salayandia, Jeffrey F Thompson, and Lisa Y Yang, et al. "Attenuation of Acute Stroke Injury in Rat Brain by Minocycline Promotes Blood–Brain Barrier Remodeling and Alternative Microglia/Macrophage Activation during Recovery." J Neuroinflammation 12 (2015): 26.
[Crossref] [Google Scholar] [PubMed]
- Colombo, Emanuela, Rosaria Pascente, Daniela Triolo, and Claudia Bassani, et al. "Laquinimod Modulates Human Astrocyte Function and Dampens Astrocyte-Induced Neurotoxicity during Inflammation." Molecules 25 (2020): 5403.
[Crossref] [Google Scholar] [PubMed]
- Zhang, Xue, Jianing Jin, and Anmu Xie. "RETRACTED ARTICLE: Laquinimod Inhibits MMP+ Induced NLRP3 Inflammasome Activation in Human Neuronal Cells." Immunopharmacol Immunotoxicol 42 (2020): 264-271.
[Crossref] [Google Scholar] [PubMed]
- Cervantes-Barragan, Luisa, Jiani N Chai, Ma Diarey Tianero, and Blanda Di Luccia, et al. "Lactobacillus reuteri Induces Gut Intraepithelial CD4+ CD8αα+ T Cells." Science 357 (2017): 806-810.
[Crossref] [Google Scholar] [PubMed]
- Cervantes-Barragan, Luisa, Jiani N Chai, Ma Diarey Tianero, and Blanda Di Luccia, et al. "Lactobacillus reuteri Induces Gut Intraepithelial CD4+ CD8αα+ T Cells." Science 357 (2017): 806-810.
- Ralph, Stephen J, Andrew Weissenberger, Ventzislav Bonev, and Liam D King, et al. "Phase I/II Parallel Double-Blind Randomized Controlled Clinical Trial of Perispinal Etanercept for Chronic Stroke: Improved Mobility and Pain Alleviation." Expert Opin Investig Drugs 29 (2020): 311-326.
[Crossref] [Google Scholar] [PubMed]
- Zhu, Shilin, Siyuan Tang, and Feng Su. "Dioscin Inhibits Ischemic Strokeâ??Induced Inflammation Through Inhibition of the TLR4/Myd88/NFâ??κb Signaling Pathway in A Rat Model." Mol Med Rep 17 (2018): 660-666.
[Crossref] [Google Scholar] [PubMed]
- Nazari, Maryam, Somaye Keshavarz, Ali Rafati, and Mohammad Reza Namavar, et al. "Fingolimod (FTY720) Improves Hippocampal Synaptic Plasticity and Memory Deficit in Rats Following Focal Cerebral Ischemia." Brain Res Bull 124 (2016): 95-102.
[Crossref] [Google Scholar] [PubMed]
- Qin, Chuan, Wen-Hui Fan, Qian Liu, and Ke Shang, et al. "Fingolimod Protects against Ischemic White Matter Damage by Modulating Microglia Toward M2 Polarization Via STAT3 Pathway." Stroke 48 (2017): 3336-3346.
[Crossref] [Google Scholar] [PubMed]
- Ziqing, Zhang, Liu Yunpeng, Liu Yiqi, and Wang Yang. "Friends or Foes: The Mononuclear Phagocyte System in Ischemic Stroke." Brain Pathol 33 (2023): e13151.
[Crossref] [Google Scholar] [PubMed]
- Xu, Jinghui, Liying Zhang, Mingyue Li, and Xiaofei He, et al. "TREM2 Mediates Physical Exercise-Promoted Neural Functional Recovery in Rats with Ischemic Stroke via Microglia-Promoted White Matter Repair." J Neuroinflammation 20 (2023): 50.
[Crossref] [Google Scholar] [PubMed]
- Sibat, Humberto Foyaca. "Bilateral Putaminal Haemorrhage and Blindness in Times of the Coronavirus Pandemic and Dysbiosis: Case Report and Literature Review." Clin Schizophr Relat Psychoses 15 (2021): 1-14.
[Google Scholar] [PubMed]
- Ajmo Jr, Craig T, Lisa A Collier, Christopher C Leonardo, and Aaron A Hall, et al. "Blockade of Adrenoreceptors Inhibits the Splenic Response to Stroke." Exp Neurol 218 (2009): 47-55.
[Crossref] [Google Scholar] [PubMed]
- Jin, Rong, Xiaolei Zhu, Lin Liu, and Anil Nanda, et al. "Simvastatin Attenuates Stroke-Induced Splenic Atrophy and Lung Susceptibility to Spontaneous Bacterial Infection in Mice." Stroke 44 (2013): 1135-1143.
[Crossref] [Google Scholar] [PubMed]
- Wang, Zhe, Da He, Ya-Yue Zeng, and Li Zhu, et al. "The Spleen May be an Important Target of Stem Cell Therapy for Stroke." J Neuroinflammation 16 (2019): 20.
[Crossref] [Google Scholar] [PubMed]
- Zhou, Sheng-Yu, Zhen-Ni Guo, Yi Yang, and Yang Qu, et al. "Gut-Brain Axis: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke through Immune Functions." Front Neurosci 17 (2023): 1081347.
[Crossref] [Google Scholar] [PubMed]
- Deng, Jiahong, Chenghan Chen, Shuaishuai Xue, and Daoqing Su, et al. "Microglia-Mediated Inflammatory Destruction of Neuro-Cardiovascular Dysfunction after Stroke." Front Cell Neurosci 17 (2023): 1117218.
[Crossref] [Google Scholar] [PubMed]
- Saand, Aisha R., Fang Yu, Jun Chen, and Sherry HY Chou. "Systemic Inflammation in Hemorrhagic Strokes–A Novel Neurological Sign and Therapeutic Target?." J Cereb Blood Flow Metab 39 (2019): 959-988.
[Crossref] [Google Scholar] [PubMed]
- Wang, Qing, Jialing Zheng, Sven Pettersson, and Richard Reynolds, et al. "The Link between Neuroinflammation and the Neurovascular Unit in Synucleinopathies." Sci Adv 9 (2023): eabq1141.
[Crossref] [Google Scholar] [PubMed]
- Cao, Fanfan, Ying Wang, Yuting Song, and Fengxia Xu, et al. "Celastrol Treatment Ameliorated Acute Ischemic Stroke-Induced Brain Injury by Microglial Injury Inhibition and Nrf2/HO-1 Pathway Activations." Biomed Res Int 2023 (2023): 1076522.
[Crossref] [Google Scholar] [PubMed]
- Shen, Xueyang, Mingming Li, Kangmei Shao, and Yongnan Li, et al. "Post-Ischemic Inflammatory Response in the Brain: Targeting Immune Cell in Ischemic Stroke Therapy." Front Mol Neurosci 16 (2023): 1076016.
[Crossref] [Google Scholar] [PubMed]
- Xue, Yunwen, Minhua Gu, Cuilan Chen, and Yujian Yao, et al. "Apolipoprotein E Mimetic Peptide COG1410 Alleviates Bloodâ??Brain Barrier Injury in a Rat Model of Ischemic Stroke." Mol Med Rep 27 (2023): 85.
[Crossref] [Google Scholar] [PubMed]
- McLeod, Ruth Mae, Ted S Rosenkrantz, Roslyn Holly Fitch, and Rachel R Koski. "Sex Differences in Microglia Activation in a Rodent Model of Preterm Hypoxic Ischemic Injury with Caffeine Treatment." Biomedicines 11 (2023): 185.
[Crossref] [Google Scholar] [PubMed]
- Zeng, Jinsong, Tingting Bao, Kailin Yang, and Xiaofei Zhu, et al. "The Mechanism of Microglia-Mediated Immune Inflammation in Ischemic Stroke and the Role of Natural Botanical Components in Regulating Microglia: A Review." Front Immunol 13 (2023): 1047550.
[Crossref] [Google Scholar] [PubMed]
Citation: Valdes, Lourdes de Fatima Ibanez and Humberto Foyaca Sibat. “New Hypotheses on the Role of Microglias in Ischemic Reperfusion Injury Secondary to Neurocysticercosis.” Clin Schizophr Relat Psychoses 17 (2023).
Copyright: © 2023 Valdes LFI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.