Case Study - Clinical Schizophrenia & Related Psychoses ( 2022) Volume 0, Issue 0
Neuromyelitis Optica Spectrum Disorder Associated with Arterial Hypertension and Pityriasis Versicolor. A Novel Presentation?
Sibi Joseph, Lourdes de Fátima Ibañez-Valdé and Humberto Foyaca-Sibat*Humberto Foyaca-Sibat, Departamen of Neurology, Nelson Mandela Academic Central Hospital (NMACH), Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 25-Jul-2022, Manuscript No. CSRP-22-70310; Editor assigned: 29-Jul-2022, Pre QC No. CSRP-22-70310 (PQ); Reviewed: 12-Aug-2022, QC No. CSRP-22-70310; Revised: 19-Sep-2022, Manuscript No. CSRP-22-70310 (R); Published: 29-Aug-2022, DOI: 10.3371/CSRP.JSDL.082922
Abstract
Neuromyelitis Optica Spectrum Disorder (NMOSD) is a rare antibody-mediated disease of the CNS causing astropathy and leading to demyelination. It typically does affect the optic nerves and the spinal cord, especially in long segments, but other features are helpful for the diagnosis.
The disease has been reported to have associations with dermatological conditions such as pruritus and eczematous changes; this is thought to be because the skin and the CNS both have the same water channels, AQP4. Other reports have been made associating NMOSD with hypertension, as a side effect of the drugs or as comorbidity.
We report a young lady who showed NMOSD and previously unreported skin condition in association with it: Pityriasis versicolor and hypertension in the young simultaneously. Based upon our literature review, we have not seen these three conditions in any one patient and hence felt it would be a noteworthy addition to the body of knowledge of this rare disorder.
Keywords
Neuromyelitis optica spectrum disorder • Transverse-longitudinal myelitis • Optic neuritis • Antibody-Mediated disease • Astropathy • Demyelinating disorder • Pityriasis versicolor
Abbreviations
AC: Anterior Chamber; APS: Area Postrema Syndrome; AQP-4: Aquaporin-4; AZA: Azathioprine; BBB: Blood-Brain Barrier; BMI: Body Mass Index; CDR: Cup Disc Ratio; CN: Cranial Nerves; CNS: Central Nervous System; CSF: Cerebrospinal Fluid; CT: Computed Tomography; EAAT2: Excitatory Amino Acid Transporter 2; ESR: Erythrocyte Sedimentation Rate; FBC: Full Blood Count; FC: Finger Counting; FDA: Food And Drug Administration; HIV: Human Immunodeficiency Virus; IL-6: Interleukin- Six; IV: Intravenous; LETM: Longitudinally Extensive Transverse Myelitis; LFT: Liver Function Tests; LL: Lower Limbs; MMF: Mycophenolate Mofetil; MOG: Myelin Oligodendrocyte Glycoprotein; MRI: Magnetic Resonance Imaging; MS: Multiple Sclerosis; NLP: No Light Perception; NMO: Neuromyelitis Optica; NMO-IgG: Neuromyelitis Optica-Immunoglobulin G; NMOSD: Neuromyelitis Optica Spectrum Disorder; OCT: Ocular Cohesive Tomography; OD: Ocular Dextro; ON: Optic Neuritis; OS: Ocular Sinister; SLE: Systemic Lupus Erythematosus; RTX: Rituximab; UL: Upper Limbs; VA: Visual Acuity
Introduction
Background
Neuromyelitis optica spectrum disorder (NMOSD) is the term given to a rare autoimmune disorder that is characterized by inflammation and demyelination of the central nervous system (CNS).
Though typically it was thought to affect the optic nerves and the spinal cord, causing optic neuritis and myelitis, respectively, recent advances have led us to appreciate that the clinical manifestations are much more diversein addition to those already mentioned to include the brainstem.
While NMOSD does occur globally, it has a higher prevalence among blacks, Asians, and Caucasians in descending order [1].
The aetiology of NMOSD, despite much research, is still unclear; however, recent investigations on immunopathogenesis brought to light the Antibodies Aquaporin-4 (AQP-4) and myelin oligodendrocyte glycoprotein (MOG), which have contributed significantly to the understanding and thus management of this disease spectrum [1-3].
There have been reported cases of NMOSD with other autoimmune systemic diseases such as Sjogren syndrome, Systemic Lupus Erythematosus (SLE) [2], rare cutaneous manifestations [4,5] and even hypertension [6]. Here we report a patient with NMOSD associated with cutaneous disease and hypertension as her presenting complaints.
Literature Review
Definition and history
Neuromyelitis Optica (NMO) was first reported in the literature in 1870 by Allbutt, et al. [7]. Then in 1894, Eugene Devic and his doctoral student Fernand Gault described the first series of cases, sixteen of which were thus also referred to as Devic’s disease [8]. Finally, in 2004, the putative antigenic target, the aquaporin-4 water channel, was discovered and would shed light on the pathophysiology and eventually leading to NMO being removed from the umbrella of Multiple Sclerosis (MS) and got a new banner title subsequently-NMOSD [8].
NMOSD is a well-known autoimmune disease of the Central Nervous System (CNS) associated with a characteristic pattern of astrocyte dysfunction and loss, resulting in secondary demyelination and neurodegeneration [9].
Usually, it is associated with a severe poor prognosis, and the disease though initially to be monophasic. Many published of cases since its discovery have indicated that there is an occurrence of frequent and severe relapses that contribute to disability over time [8,9].
Pathogenesis
AQP4 is localized mainly in the foot processes of the astrocytes at the blood-brain barrier (BBB). Other parts where they are found include the collecting ducts of the kidneys, parietal cells in the stomach, airways, and secretory glands and the skeletal muscles [8].
A serum biomarker called Neuromyelitis Optica (NMO)-immunoglobulin G (IgG), which targets Astrocytic (AQP4) water channels [10] and cell membranes expressing AQP4, was discovered and attributed to demyelination if IgG targets astrocytic Ranvier node processes [11]. AQP4 antibodies are produced chiefly in the periphery (though not exclusively), and their pathogenic effects are primarily seen in the CNS, especially in the NMOSD.
NMO-IgG increases the BBB permeability by accessing the intrathecal compartment behind the BBB and allowing the action of a non-AQP4 endothelial-specific immunoglobulin [12]. In the laboratory studies conducted by Takeshita, they reported that NMO-IgG binding to the astrocyte AQP4 selectively induced its internalization and the production of IL-6. The astrocytes also express IL-6 in neuroinflammation, downregulating endothelial cells functioning tight junction molecules and decreasing barrier function [12].
Specific AQP4 depletion, with or without concomitant myelin loss, localized vasculocentric IgG, IgM, and complement deposits, significant oedema, and inflammation are features of characteristic CNS lesions. Astrocytic membranes are still intact without complement, but AQP4 is endocytosed, resulting in the simultaneous loss of Na+-dependent glutamate transport and excitatory amino acid transporter 2 (EAAT2) [10].
The binding of NMO-IgG to astrocytic AQP4 starts several potentially neuropathogenic pathways, including complement activation, downregulation of AQP4 and EAAT2, and disturbance of glutamate homeostasis [10].
As a result, the astrocyte is rendered helpless, ultimately losing support for neighbouring cells, including oligodendrocytes and neurons, following granulocyte infiltration, oligodendrocyte destruction and demyelination occur [8].
Clinical features
The affected regions of the CNS are seen where AQP4 is most abundantly expressed such as spinal cord (Longitudinally Extensive Transverse Myelitis (LETM)), Optic Nerve (optic neuritis), dorsal aspect of medulla oblongata leading to Area Postrema Syndrome (APS), Brainstem (Acute Brainstem Syndromes), and Thalamus/Hypothalamus (Acute Diencephalic Syndromes, e.g., Symptomatic Narcolepsy). Attacks are frequently severe and often reach a nadir in less than a week.
LETM is the most typical presentation of NMOSD and is rare in multiple sclerosis. It typically consists of inflammation affecting the central grey matter, extending over three or more contiguous vertebral bodies. LETM often present like paraplegia or tetraplegia depending on the spinal cord’s involvement. A sensory disorder and bladder involvement are useful distinguishing features from other causes of rapid evolving weakness such as Guillain-Barré syndrome.
Optic neuritis (ON): Typically present as longitudinally extensive lesions with a preference for posterior segments of the nerve close to the ventral aspect of the chiasm. ON usually affect the optic nerve bilaterally associated to severe loss of visual acuity, ocular pain, and poor recovery (<6/60) which serve to identify the ophthalmological disorder.
In most of cases, the area postrema syndrome (APS) is characterized by intractable nausea, hiccups and vomiting due to swelling to the emetic reflex centre situated in the rhomboid fossa of the fourth ventricle. Some patients present gastroenteritis or cyclical vomiting syndrome at the beginning of the NMOSD manifestations.
Acute brainstem syndromes: Overlaps with APS but also include patients who present with oculomotor dysfunction (e.g., diplopia and nystagmus), or other cranial nerve palsies, depending on location.
Hypothalamic periventricular regions contain highest APQP4 expressions and bilateral involvement may affect hypothalamic hypocretin neuronal function.
Several cases of narcolepsy have been reported with diencephalic lesions and low cerebrospinal fluid (CSF) hypocretin levels. Although NMO might suggest exclusive spinal cord and optic nerve inflammation, brain involvement can be seen in 60% of patients, though most observed changes are nonspecific. Although, cerebral lesions may be asymptomatic in many cases epileptic seizures, corticospinal tract lesions and encephalopathy can be seen and can be identify as enhancement lesions in MRI with contrast and even mistaken for a primary brain malignancy. Because of high expression of AQP4 in the peri ependymal areas around the ventricular system then characteristic lesions on the regions can be observed.
Diagnostic criteria
As a general agreement, additional MRI requirements for NMOSD without AQP4-IgG and NMOSD with unknown AQP4-IgG status [12].
Acute ON requires brain MRI showing normal findings or only nonspecific white matter lesions, OR general optic nerve MRI with T2- hyperintense lesion or T1-weighted gadolinium-enhancing lesion extending over ON length or involving optic chiasm.
Acute myelitis: requires associated intramedullary MRI lesion extending over >3 contiguous segments (LETM) OR >3 adjacent components of focal spinal cord atrophy in patients with a history compatible with acute myelitis.
Diagnosis of APS is accepted only if associated dorsal medulla lesions are present. Acute brainstem syndrome requires associated peri ependymal brainstem lesions.
NMOSD and dermatological diseases
According to Misery, the literature has poorly reported NMOSD and dermatological issues. However, they acknowledge that NMOSD can cause neuropathic pruritus and be associated with other autoimmune conditions [5]. Neuropathic pruritus is usually associated with peripheral nerve disorders; however, NMOSD is one of the few central nervous system disorders that leads to it. In addition, this pruritus is associated with allodynia, hyperesthesia, and hypoesthesia, among others [5]. Other cutaneous manifestations include:
• Reynaud’s phenomenon.
• Xerosis.
• Secondary eczematous changes usually complicate anhidrosis.
• Erythematous rash in the presence of rotavirus infection.
Some explanation is that the pathogenesis of the diseases shares some similarities but is not thoroughly investigated and clear. For example, the mammalian skin also contains Aquaporin-4 channels, and their damage might lead to poor wound healing and cutaneous tumorigenesis, but most interestingly, atopic dermatitis [4].
NMOSD and arterial hypertension
A study by Chen and colleagues found that in the acute phase of NMOSD, patients did high blood pressure (BP) [6]. In comparison, another study considered hypertension one of the most common comorbidities to NMOSD [7]. Both studies did look at different factors such as the use of certain medications like glucocorticoids in the acute phase, BMI, and others. While no conclusive evidence exists, many correlations are evident, and many more studies are warranted without doubt [6,7].
Management
The treatment must be considered in two ways: acute management and long-term therapy.
The acute treatment of an NMOSD attack is the use of IV methylprednisolone for five days which is then converted to oral prednisolone given at 1 mg/kg and then gradually tapered over months. All the literature advocates early treatment, especially for neurological deficits. Nevertheless, in patients with no improvement to oral corticosteroids steroids then IV methylprednisolone and five cycles of plasma exchange (PLEX) are a viable option [13].
There is evidence that PLEX is effective as a first-line treatment for relapses, especially myelitis, but prospective randomized trials are necessary to support this claim [14]
The prognosis for relapsing NMO is poor; medication, frequently involving immunosuppression, is required as early in the disease’s progression as feasible to prevent attack-related impairment [15]. For relapse prevention, efficient immunosuppression is the gold standard of therapy. In individuals with NMO and NMOSD, initial drugs of choice are rituximab, Mycophenolate Mofetil (MMF), and, to a lesser extent, azathioprine which considerably lowers relapse rates. Patients who fail to achieve remission are switched from one drug to another [16].
Abbadessa, reviewed all the studies aimed at the retreatment of NMOSD. They reported that the retreatment schedule for RTX in NMOSD has not yet been determined, and the presented studies indicated that CD27+ B cells might be helpful biomarkers to guide retreatment in AQP4- IgG positive patients. They recommended further studies to identify factors influencing anti-CD20 therapy effectiveness to adjust the dosage and treatment schedules [17].
The therapy of NMOSD patients with RTX, MMF, and AZA is associated with fewer relapses and an improvement in disability, according to the findings of a systematic review and meta-analysis by Jia Ma, there was no significant difference in the effects of the three drugs on lowering Expanded Disability Status Scale scores, but RTX significantly decreased annual relapse rate in comparison to the other drugs [18].
According to the PREVENT trial, individuals with anti-AQP4 immunoglobulin G-positive (AQP4+) NMOSD have a lower adjudicated relapse risk when taking eculizumab, a terminal complement inhibitor and consistently more effective than placebo at reducing the probability of relapse in a variety of clinically pertinent AQP4+ NMOSD patient subgroups in PREVENT, with no noticeable increase in the rate of serious infection [19]. It was the first treatment approved by the United States Food and Drug Administration (FDA) to manage NMOSD [20,21].
Should consider general management measures as well. For example, when the patient remains bedridden, thromboprophylaxis must be administered. In addition, there are chances that the patient may experience neuropathic pain and tonic spasms following transverse myelitis, and in those situations, low dose carbamazepine has been useful [22]. A multidisciplinary approach is also advocated for, especially as we have seen that NMOSD can occur with other diseases, viz dermatological conditions, hypertension, and connective tissue disorders, to mention a few; the ophthalmologists must be involved for their invaluable input as well. However, the multidisciplinary approach extends not only to managing physicians but also to the extent of physiotherapists and occupational therapists for physical rehabilitation. In addition, social services and psychologists are vital, especially as many patients and their loved ones need to fully understand the weight of the disease’s burden and the importance of compliance and regular follow-ups with the neurologist.
Case Presentation
We present a 22-year-old female of African descent who is right- handed, who was referred to our hospital from a peripheral district hospital with a two days history of a sudden loss of vision bilaterally, and inability to ambulate; she had also assessed as a newly diagnosed hypertensive with repeated readings ranging from 180/113-215/120.
The patient was previously well and had no ophthalmoplegia, headaches, nausea, vomiting, and no bladder, bowel issues or even any illnesses, vaccinations, or surgeries in the preceding weeks. She is nulliparous and does not use contraceptives. No history of weight gain or trauma recently. She has no positive family history, and she is of sober habits.
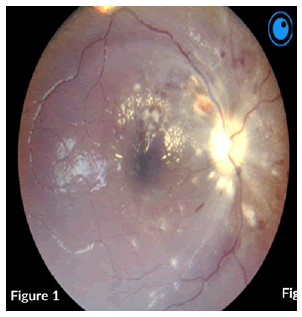
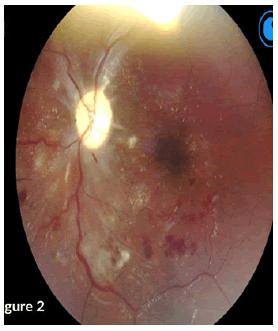
On examination, a bed-bound patient with hypopigmented patches and scaling involving the face, trunk, and upper arms. She had high blood pressure of 159/110 heart rate of 60 bpm. A focused CNS examination revealed an awake patient, oriented to time, place, person, and situation, with intact higher functions. Nil meningism and all other cranial nerves except CN II were normal. Figures 1 and 2 shows abnormalities on fundoscopy.
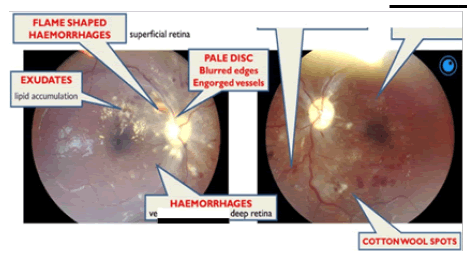
CN II OD VA-NPL, dense and quiet anterior chamber (AC) with a pharmacodilated pupil of 6.5 mm. Fundoscopy showed grade III disc oedema with engorged vessels, retinal haemorrhages, flame-shaped haemorrhages superiorly and cotton wool spots around the disc and macula. OS VA – FC at 1 m, dense and quiet AC, with a Pharmacodilated pupil of 6.5 mm, fundoscopy showed no disc oedema, cotton wool spots and exudates around the disc and macula, engorged vessels, and flameshaped haemorrhages (Figure 3).
The motor exam showed Upper Limbs (UL) were normal; however, Lower Limbs (LL), while the inspection was average power, was 4/5 proximally and 3/5 distally, with spastic tone (hypertonia) and 4+ reflexes (Hyperreflexia with clonus) in the ankles. Babinski’s sign was present bilaterally. Sensory and Coordination were intact globally, and gait could not be assessed.
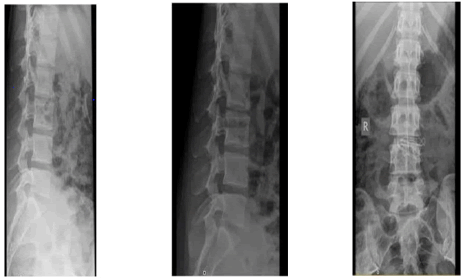
Plain lumbosacral X-rays showed no remarkable abnormalities (Figure 4).
Results and Discussion
Blood work of note
HIV ELISA negative, negative antibody screen, AQP-4 IgG Negative. Lumbar puncture with opening pressure of 18 cm H2O, CSF protein of 0.14 mmol/L, CSF IgG 679 (Borderline), the rest of the investigation results can be seen in Table 1.
| Variable | Patient value | Normal range |
|---|---|---|
| White cell count | 7.10 × 109/L | 3.9-12, 6 × 109/L |
| Hb | 13.4 g/dl | 12-15 g/dl |
| Platelets | 379 × 109/L | 186-454/L |
| Sodium | 140 mmol/L | 136-145 mmol/L |
| Potassium | 4.1 mmol/L | 3.5-5.1 mmol/L |
| Chloride | 101 mmol/L | 98-105 mmol/L |
| Urea | 5.8 mmol/L | 2.1-7.1 mmol/L |
| Creatinine | 68 µmol/L | 48-90 µmol/L |
| Calcium | 2.0 mmol/L, | 2.15-2.5 mmol/L |
| Magnesium | 0.74 mmol/L, | 0.63-1.05 mmol/L |
| Phosphate | 1.32 mmol/L | 0.78-1.42 mmol/L |
| Ferritin | 22 ng/ml | 11-307 microgram/L |
| C-reactive protein | 1 mg/L | <3 mg/L |
| Erythrocyte sedimentation rate | 8 mm/h | 0-10 mm/hr |
| Total protein | 74 g/L | 60-78 g/L |
| Total Bilirubin | <9 µmol/L | 5-21 µmol/L |
| Alkaline phosphatase | 65 U/L | 42-98 U/L |
| Aspartate transaminase | 26 U/L | 13-35 U/L |
| Alanine transaminase | 30 U/L | 7-35 U/L |
| Total cholesterol | 3.71 mmol/L | <4.5 mmol/L |
| HbA1C | 6.10% | <7% |
| International normalized ratio | 1.01 | 1 |
| D-dimer | 0.1 mg/L | 0.00-0.25 mg/L |
| Rheumatoid factor | 10 IU/ml | <20 IU/L |
| Vitamin B12 | 231 pmol/L | 145-569 pmol/L |
| Thyroid stimulating hormone | 0.99 IU/mL | 0.27-4.2 Miu/l |
| Anticardiolipin | negative | |
| antibody Protein S | 66 IU/dl | 55-123 IU/dl |
| Protein C | 111 IU/dl | 70-130 IU/dl |
| Angiotensin converting enzyme | 38 IU/L | 8-53 IU/L |
| Interleukin-6 | It was no performed | |
| Anti-streptolysin O titer | 88 IU/ml | <200 IU/L |
| Toxoplasmosis Gondi IgG antibody | Negative | |
| Cytomegalovirus IgG antibody | Negative | |
| Rubella IgG antibody | Negative | |
| Rubella IgM antibody | Negative | |
| Cytomegalovirus IgM antibody | Negative | |
| C3 | 1.2g/L (0.9-1.8 g/L) | |
| C4 | 0.2g/l (0.1-0.4 g/L) | |
| Antinuclear antibody | Negative | |
| Anti-double strand DNA | Negative | |
| antibody Anti-RNP antibody | Negative |
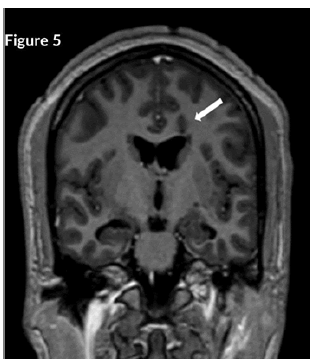
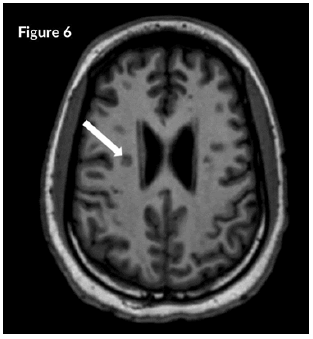
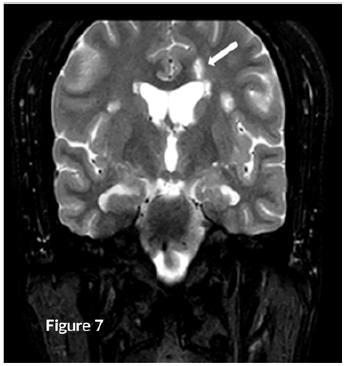
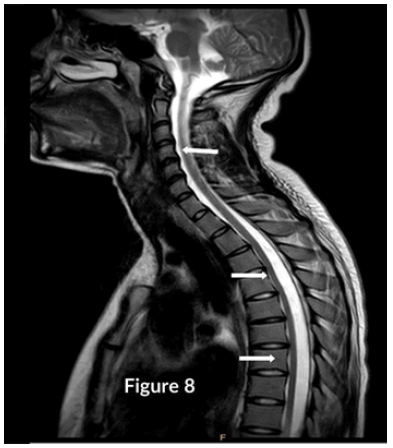
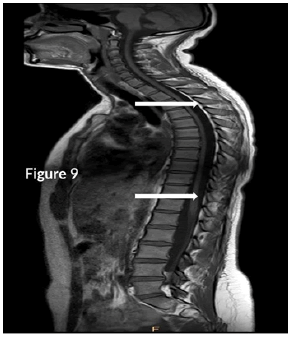
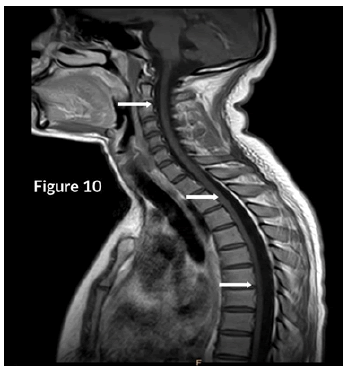
CT Brain was grossly within normal parameters. MRI brain and spine: showed bilateral periventricular and juxtacortical and white matter high signal on T2/Flair and low on T1W, no enhancement post-contrast. Similar lesions were noted in the temporal lobe. Images of the spinal cord confirmed generalized helicoid atrophy with no abnormal cord signal (Figures 5-10)
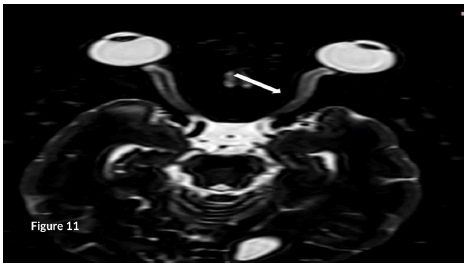
We performed an MRI of the ON which confirmed lesions in the intra- orbital segment of the ON, which appeared swollen, with a high T2 signal. These high T2 signal persisted, and the ON appeared atrophied rather than swollen (Figure 11).
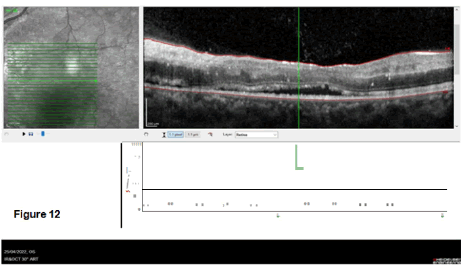
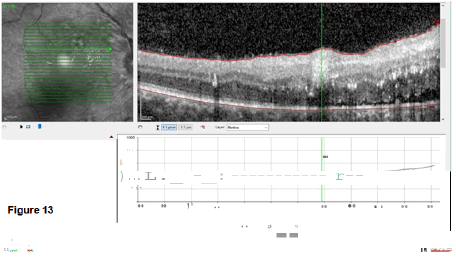
The last images study done was the Optical Coherence Tomography (OCT) as a non-invasive imaging test. Light waves to take cross-section pictures of retina’s patients were used to see each of the retina’s distinctive layers which allowed to map and measure their thickness (Figures 12 and 13).
The OCT OD showed a subretinal fluid collection with macular oedema of about 444 mm and exudates and haemorrhages. OS similar findings, but the macular oedema was only 303 mm. The lesions were distributed in space and time because no restricting or enhancing lesion was visualized.
Patient was diagnosed with NMOSD, young hypertension and pityriasis versicolor. She was started on three days of IV methylprednisolone at a dose of 1 g/day, then converted to oral prednisone of 40 mg/day. Then began on Azathioprine (AZA) at 50 mg daily for a week, and then once there was no derangement in the FBC, differential count or LFTs, the dosage was increased to 100 mg daily. She was also started on Rituximab 375 mg/m2 weekly for four weeks while constantly monitoring the above parameters and hepatitis screening. Patient was then referred to the physicians who started her on antihypertensives, who worked her up for causes of hypertension and could not find any metabolic, connective tissue disease or renal cause. The dermatology department started her on antifungals as well. She also received intensive physiotherapy and occupational therapy and organized a review with the social worker services.
Four weeks post the treatment, the patient was VA OD still NPL with a pale disc, CDR of 0.3, resolving haemorrhages and minimal cotton wool spots and exudates. OS VA of 0.05, pink disc, CDR of 0.3 with macular oedema. The motor exam showed that all signs had resolved, and the patient could ambulate without support. OCT findings had also improved significantly with OD macular thickness of 182 mm and OD 162 mm.
She was subsequently discharged to continue with her AZA, prednisone, and supplements. She is also receiving physiotherapy as an outpatient. especially the neurologist (for obvious reasons) but also for the internal medicine physicians and dermatologists. While it would be easy to miss the diagnosis, we made had the patient’s issues been dealt with in silos, a high index of suspicion needs to be maintained to understand the possible underlying pathology fully.
While we did not find any study linking fungal skin infections associated with NMOSD characterized by brain, ON and spinal cord demyelinating lesios, we found viral infections implicated; thus, further scrutiny is warranted to establish if the fungal infection would damage the AQP4 channels.
It is the first case where a patient has both cutaneous and hypertension associated with NMOSD.
Declarations
Ethical considerations
This review was not considered ethical approval because all the data were extracted from previously published articles. Nevertheless, we obtained signed written consent from our patient for publishing her clinical information and images. Therefore, the Institutional Ethical Committee did not consider this study for additional ethical approval.
Competing Interests
The authors have not any conflict of interest to disclose. We declare no commercial or financial relationships construed as a potential conflict of interest.
Funding
The authors declared that they never received any financial support or personal collaboration that could have influenced the results reported in this paper.
Declaration of Anonymity
The authors certify that they did not reveal the names, surnames, initials of the patient. Alternatively, other identity issues of this case in this publication and complete anonymity are guaranteed.
Availability of Data and Material
The data that support the findings of this study are available on reasonable request from the corresponding author.
Acknowledgements
We sincerely thank Dr Mompie for his enthusiastic support and radiological studies done.
References
- Jeyalatha, Mani Vimalin, Kulandai Lily Therese, and Appakkudal Ramaswamy Anand. "An Update on the Laboratory Diagnosis of Neuromyelitis Optica Spectrum Disorders." J Clin Neurol (Seoul, Korea)18(2022): 152.
- Bollo, Luca, Carlo Santoro, Roberta Pellicciari and Damiano Paolicelli, et al. "Neuromyelitis Optica Spectrum Disorders Associated with Systemic Sclerosis: A Case Report and Literature Review." Neurol Sci 43(2022): 4015-4018.
- Wingerchuk, Dean M., Brenda Banwell, Jeffrey L. Bennett and Philippe Cabre et al. "International Consensus Diagnostic Criteria for Neuromyelitis Optica Spectrum Disorders."Neurol 85(2015): 177-189.
- Martin, Carina, Toby Maurer, and Misha M. Mutizwa. "Neuromyelitis Optica with Cutaneous Findings: Case Report and Review of the Literature." Dermatol230(2015): 289-292.
- Misery, Laurent, Steeve Genestet, and Fabien Zagnoli. "Neuromyelitis Optica and Skin."Dermatol (2022): 1-6.
- Chen, Xiaohong, Rong Fan, Fuhua Peng and Jia Liu et al. "Blood Pressure and Body Fat Percent in Women with NMOSD." Brain Behav 9(2019): e01350.
- Ajmera, Mayank R., Audra Boscoe, Josephine Mauskopf and Sean D. Candrilli et al. "Evaluation of Comorbidities and Health Care Resource use Among Patients with Highly Active Neuromyelitis Optica." J Neurol Sci 384(2018): 96-103.
- Shafiq, Irfan, Ali Saeed Wahla, Mateen Haider Uzbeck and Naureen Khan et al. "Neuromyelitis Optica Spectrum Disorder Associated with Cryptogenic Organizing Pneumonia in a Young Patient." Eur J Case Rep Intern Med 9(2022).
- Carnero Contentti, Edgar, and Jorge Correale. "Neuromyelitis Optica Spectrum Disorders: From Pathophysiology to Therapeutic Strategies." J Neuroinflammation 18(2021): 1-18.
- Hinson, Shannon R, Shanu F. Roemer, Claudia F. Lucchinetti and James P. Fryer et al. "Aquaporin-4–Binding Autoantibodies in Patients with Neuromyelitis Optica Impair Glutamate Transport by Down-Regulating EAAT2." Exp. Med. 205(2008): 2473-2481.
- Hinson, Shannon R, SJ Pittock, CF Lucchinetti and SF Roemer et al. "Pathogenic Potential of Igg Binding to Water Channel Extracellular Domain in Neuromyelitis Optica." Neurol69(2007): 2221-2231.
- Takeshita, Yukio, Birgit Obermeier, Anne C. Cotleur and Simona F. Spampinato et al. "Effects of Neuromyelitis Optica–Igg at The Blood–Brain Barrier in vitro." Neurol Neuroimmunol Neuroinflamm 4(2017).
- Kleiter, Ingo, Anna Gahlen, Nadja Borisow and Katrin Fischer et al. "Neuromyelitis Optica: Evaluation of 871 Attacks and 1,153 Treatment Courses."Ann Neurol 79(2016): 206-216.
- Weinshenker BG. What is the Optimal Sequence of Rescue Treatments for Attacks of Neuromyelitis Optica Spectrum Disorder?Ann Neurol 79(2016):204-205.
- Chia, Wei-Chia, Jian-Nan Wang, and Ming-Chi Lai. "Neuromyelitis Optica: A Case Report." Pediatr Neonatol51(2010): 347-352.
- Mealy, Maureen A, Dean M. Wingerchuk, Jacqueline Palace and Benjamin M. Greenberg et al. "Comparison of Relapse and Treatment Failure Rates Among Patients with Neuromyelitis Optica: Multicenter Study of Treatment Efficacy." JAMA Neurol 71(2014): 324-330.
- Abbadessa, Gianmarco, Giuseppina Miele, Elisabetta Maida and Giuseppe Minervini et al. "Optimal Retreatment Schedule of Rituximab for Neuromyelitis Optica Spectrum Disorder: A Systematic Review." Mult Scler Relat Disord (2022): 103926.
- Ma, Jia, Haihua Yu, Hao Wang and Xinghu Zhang et al. "Evaluation of Effect of Empirical Attack-Preventive Immunotherapies in Neuromyelitis Optica Spectrum Disorders: An Update Systematic Review and Meta-Analysis." J Neuroimmunol 363(2022): 577790.
- Palace, Jacqueline, Dean M. Wingerchuk, Kazuo Fujihara and Achim Berthele et al. "Benefits of Eculizumab in Aqp4+ Neuromyelitis Optica Spectrum Disorder: Subgroup Analyses of the Randomized Controlled Phase 3 Prevent Trial." Mult Scler Relat Disord47(2021): 102641.
- Alexion Announces Successful Phase 3 PREVENT Study of Soliris® (Eculizumab) in Patients with Neuromyelitis Optica Spectrum Disorder (NMOSD) | Business Wire. Accessed (2022).
- FDA Approves First Treatment for Neuromyelitis Optica Spectrum Disorder, A Rare Autoimmune Disease of The Central Nervous System | FDA. Accessed (2022).
- Palace, Jackie, Isobel Leite, and Anu Jacob. "A Practical Guide to the Treatment of Neuromyelitis Optica." Pract Neurol 12(2012): 209-214.
Citation: Joseph, Sibi, Lourdes de Fátima Ibañez-Valdés and Humberto Foyaca-Sibat. "Neuromyelitis Optica Spectrum Disorder Associated with Arterial Hypertension and Pityriasis Versicolor. A Novel Presentation?." Clin Schizophr Relat Psychoses 16S (2022). Doi: 10.3371/CSRP.JSDL.082922
Copyright: © 2022 Joseph S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.