Research - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Mutual Prodrug of 5-Ethynyluracil and 5-Fluorouracil: Synthesis and Pharmacokinetic Profile
Yasser Fakri Mustafa*, Mahmood Khudhayer Oglah, Moath Kahtan Bashir, Eman Tareq Mohammed and Raghad Riyadh KhalilYasser Fakri Mustafa, Department of Pharmaceutical, Mosul University, Nineveh, Iraq, Email: Dr.yassermustafa@uomosul.edu.iq
Received: 04-Aug-2021 Accepted Date: Aug 18, 2021 ; Published: 25-Aug-2021
Abstract
The oral administration of the standard cytotoxic agent 5-fluorouracil is extensively limited in the last three decades. This restriction is due to the drug's uneven intestinal absorption due to the changeable activity of dihydropyrimidine dehydrogenase, an enzyme found in the intestinal mucosa. A prodrug containing 5-fluorouracil and 5-ethynyluracil was developed in this study to allow mutual release of these two active medicines via a lactonizationfacilitated release mechanism. Using coumarin as a precursor, the synthesis of the target prodrug was carried out in seven stages. The chemical backbones of the synthesized intermediate molecules and the target prodrug were verified using spectra acquired from several spectrophotometers,including FTIR, 1H-NMR, and 13C-NMR. In the HCl- (pH 1.2) and phosphate- (pH 6.8) buffers, the target prodrug's chemical stability was studied. Human serum was also used to test the prodrug's ability to release its active components. Chemical stability tests revealed that the targeted prodrug had significant stability in the HCl-buffer, with a t1/2 of 33.18 hours, and in the phosphate-buffered saline, with a t1/2 of 18.14 hours, according to pseudo-first-order kinetics. Furthermore, the prodrug may liberate the two active compounds in human serum with a t1/2 of 4.62 hours using zeroorder kinetics. The authors came to the conclusion that the target prodrug might be a good mutual prodrug for oral ingestion of 5-fluorouracil and 5-ethynyluracil.
Keywords
Cyclization •Pharmacokinetic •Chemical •Kinetics
Introduction
5-Fluorouracil (FU) has been used to treat a variety of cancer morphologies since it was first designed and synthesized in 1957 [1]. Nonetheless, due to its high frequency of adverse effects, poorer target ability, and low tumor sensitivity due to established resistance, the chemotherapeutic use of this tumor-fighting agent is being avoided [2,3]. Several advancements have been studied to control these hurdles, including adjusting administration programs, adjusting metabolic fates, creating and synthesizing novel fluoro-pyrimidines, and using diverse prodrug tactics [4- 7].
Inside the viable cell, FU must be transformed via different metabolic pathways and steps into active forms since it is a prodrug [8]. The enzyme named dihydropyrimidine dehydrogenase and housed in the liver (DPDE) accounts for the basis of extracellular destruction of the plurality of the FU dose [9].
The utility of FU as an oral cytotoxic agent has been questioned because of its unpredictable GIT absorption that subsequently results in the fluctuation of the plasma levels with significant intra- and inter-individual versions. These outcomes could attribute to the changeable potential of DPDE localized in the GIT mucosa [10,11].
To enhance the oral bioavailability of the FU chemotherapy, interfering with the negative role of DPDE through its inhibition has become a potential target [12]. Although there are many evaluated inhibitory compounds, those analogs to FU exhibited the highest potential as DPDE in activators and 5-Ethynyluracil (EU) was the best [7,13-18].
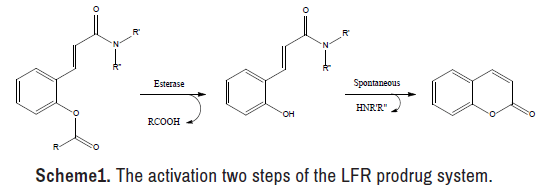
Through the past 50 years, much interest has polarized to the design and synthesis of the Lactonization-Facilitated Release (LFR) prodrugs[19-23]. This prodrug phenotype is valuable to enhance the therapeutic potency of many polar drugs by modulating their hydrophilicity or shifting their metabolism to another direction [24,25]. The LFR prodrug system, as depicted in reveals many benefits, such as the eloquent release of the active component (s) when the LFR prodrug assaults via esterase enzyme Scheme 1. Besides, the rate of releasing the active component (s) may be modified through the presence of various functional groups on the coumarin chemical nucleus [26,27]. Moreover, the last compound, coumarin, acquired since the active component (s) is free from the LFR prodrug is documented to be safe [28].
The goal of this paper is to use the LFR prodrug system to develop and synthesize a mutual prodrug. This LFR prodrug can release FU as an oral cytotoxic agent and EU as a metabolic modulator when activated. To achieve this goal, the chemical stability of the synthesized prodrug was assessed, as well as its release patterns, using two buffer systems that simulated the GIT and human plasma, respectively.
Materials and Methods
Experimental
Chemicals and solvents were obtained from foreign sources for manufacturing the LFR prodrug and its intermediate components, as well as analyzing in vitro release. Shimadzu LCMS-2020 with an electrospray ionization source was used to scan the mass spectrum, Bruker Advance DRX-400 MHz was used to identify the NMR spectra, and Bruker-Alpha ATR-FTIR was used to screen the IR spectrum to validate the chemical structures of the synthesized products. Varian UV/Visible spectroscopy was used to determine the UV spectra of the LFR prodrug and its reaction intermediates. The in vitro stability and release experiments were also monitored using the same device. The course of the reactions and also purity of products were monitored using Thin-Layer Chromatography (TLC).
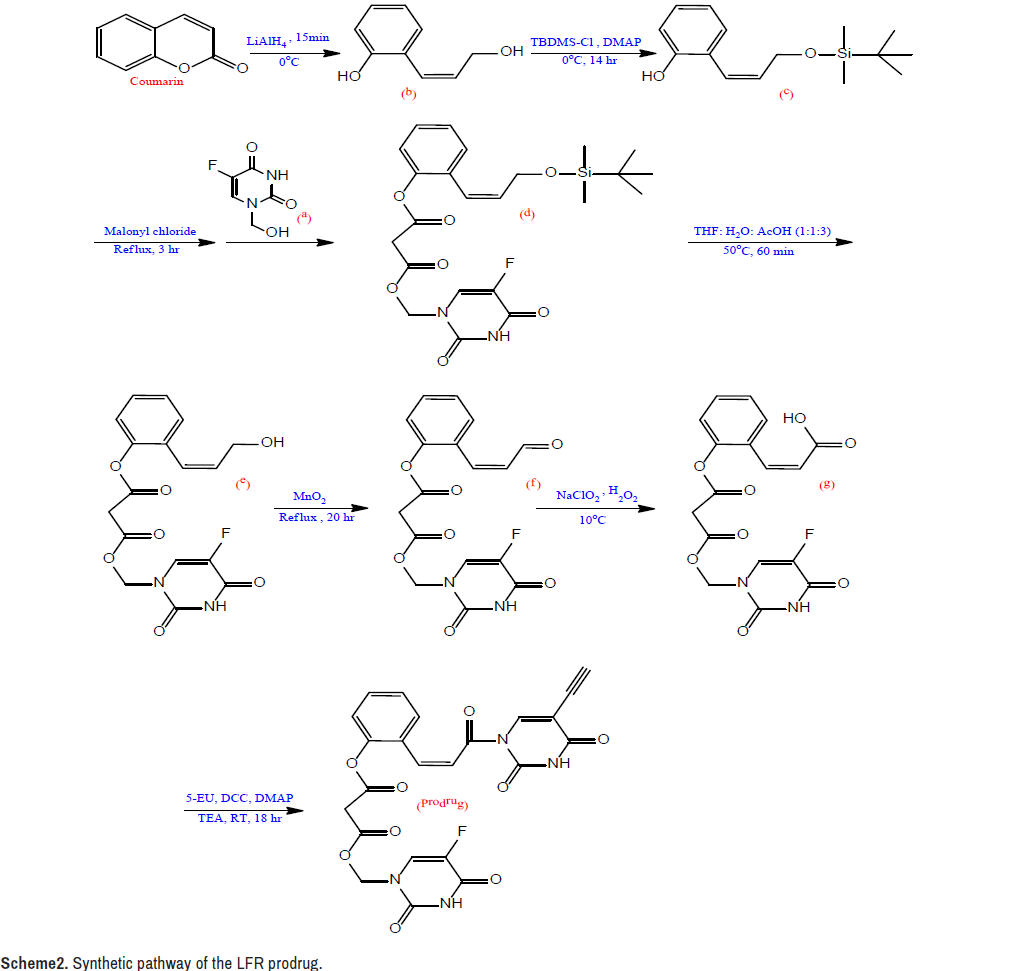
Synthesis of the intermediate a: At 60°C, a solution was produced by magnetically agitating a combination of formaldehyde (5 ml, 37%) and FU (1.04 g, 8 mmol) in 25 ml H2O for 45 minutes. Under decreased pressure, the resulting solution was evaporated to dryness, and the named product was recrystallized from EtOH [29,30].
Synthesis of the intermediate b: A solution of coumarin (25 mmol, 3.65 g) in 50 ml dry ether was mixed with a solution of lithium aluminum hydride (50 mmol, 1.9 g, LiAlH4) in 50 ml dry ether in an ice bath. The resulting mixture was agitated for 15 minutes before being treated with HCl (27 mL, 5%), yielding a pH 5 solution. The organic layer was dried over The stationary and mobile phases were precoated silica gel plates and a chloroform: Acetone (4:1) eluent system, respectively.
Chemical synthesis
The LFR prodrug was synthesized using the synthetic approach shown in Scheme 2. MgSO4, filtered, and vaporized after the crude was extracted with ether (350 ml). From EtOH, the intermediate b was recrystallized [31].
Synthesis of the intermediate c: The solution of tert-butyldimethylsilyl chloride (25 mmol, 3.79 g, TBDMS-Cl) in 35 ml dry THF was treated in an ice bath with the solution of b (22.8 mmol, 3.43 g) in 40 ml dry THF. A solution of 4-dimethylaminopyridin (34 mmol, 4.18 g, DMAP) in 40 ml dry THF was drop wise added to the resulting combination. The reaction mixture was agitated for 14 hours, then filtered and evaporated until it was completely dry. The crude was dissolved in EtOAc (50 ml) and washed with HCl (50 ml, 1 N), NaHCO3 (25 ml, 5%), and H2O in that order (25 ml).
The organic layer was filtered and evaporated after being dehydrated with MgSO4. From CHCl3, the intermediate c was recrystallized [31,32].
Synthesis of the intermediate d: Malonyl chloride (10 mmol, 1 ml) was added to a combination of c (10 mmol, 2.65 g) and a (10 mmol, 1.60 g) in 50 ml dried CHCl3. With continual stirring, the mixture was refluxed for 3 hours. TLC was used to monitor the progress of the reaction using a combination of EtOAc and ether as an eluent. The sample solution was dried over anhydrous MgSO4, condensed under minimized pressure, and purified in column chromatography with a CHCl3: EtOH (2:1) combination, yielding the named product [31,33].
Synthesis of the intermediate e: In a combination of H2O (10 ml), THF (10 ml), and acetic acid, intermediate d (4 mmol, 1.97 g) was dissolved (30 ml). The mixture was vaporized under decreased pressure after 60 minutes of stirring at 50°C. The crude was dissolved in 50 mL EtOAc, and the resulting solution was washed with 5% NaCO3 (50 mL, 5%) (50 ml). The organic layer was dehydrated with MgSO4, filtered, and evaporated at low pressure. From EtOH, the intermediate e was recrystallized [34,35].
Synthesis of the intermediate f: MnO2 (20 mmol, 1.74 g) and intermediate e (4 mmol, 1.51 g) were suspended in CHCl3 (30 ml) and refluxed for 20 hours. The heated mixture was filtered, and the solid that emerged was washed in 30 ml warm CHCl3. Under decreased pressure, the organic layer was evaporated, and the crude was dissolved in 30 ml propanone and filtered. The intended product was obtained once the solution was vaporized [36].
Synthesis of the intermediate g: An aqueous solution of the intermediate f (4 mmol, 1.50 g), NaH2PO4 (0.85 mmol, 102 mg), and H2O2 (30 percent, 4.17 mmol, 0.5 ml) in 25 ml ACN was progressively added. Using an ice-water bath, the temperature of the reaction was kept below 10°C during the addition procedure, and oxygen bubbles were seen in the reaction mixture. When the bubbles ceased forming, 0.05 g Na2SO3 was added to destroy the unreacted H2O2 and HOCl. The reaction mixture was acidified to pH 2 with 1N HCl before being extracted with 50 ml EtOAc. The organic layer was dried over MgSO4, filtered, and vaporized after being washed with 25 ml brine. The crude was dissolved in ACN and filtered after being treated with an aqueous NaHCO3 solution to achieve a pH of 6.5. As the filtrate was acidified to pH 3 with 1N HCl, the desired product was separated [37,38].
Synthesis of the LFR prodrug
5-EU ( 2 mmol, 0.27 g), DCC (2.4 mmol, 0.5 g), DMAP (0.17 mmol, 20 g), and TEA (2 mmol, 0.3 ml) were serially added to a solution of g (2 mmol, 0.78 g) in 50 ml freshly distilled DMSO put in an ice-water bath. For 18 hours, the mixture was mixed at room temperature. The reaction mixture was then treated with 5 ml MeOH and 0.5 ml acetic acid, agitated for 60 minutes, then neutralized with an aqueous NaHCO3 solution. The crude was washed with H2O after the precipitate was filtered, the filtrate was vaporized, and the filtrate was vaporized. A combination of EtOH and CHCl3 (2:1.5) was used to recrystallize the target compound [31].
In vitro kinetic studies
Chemical stability: The chemical stability of the produced prodrug was tested in two pH buffers: HCl (pH 1.2) buffer and phosphate-buffered saline buffer (pH 6.8) [39,40]. The following mathematical formula of Beer's law was used to track the reduction in prodrug concentration vs time in this investigation using UV/Visible spectroscopy: “Absorbance =∈× L × C” [41].
L is the path length of the cell holder (2 cm), C is the prodrug concentration, and ∈ is the absorbance coefficient.
A warmed LFR prodrug (5 mol) solution in 2 ml DMSO was combined with 48 ml prepared buffer solution. The timer was started, and the resulting solution was kept at 37°C in a warm water bath for preservation. The solution was then divided into a set of ten test tubes, each containing 5 ml. An individual test tube was chosen for each time interval of 0.5, 1, 1.5, 2, 2.5, 3, 3.5, or 4 hours, and its contents were mixed with 2 ml dichloromethane.
To identify the remaining concentration of the prodrug, the aqueous aliquot (2 ml) was spectrophotometrically assessed at designated λmax [42,43].
Enzymatic hydrolysis
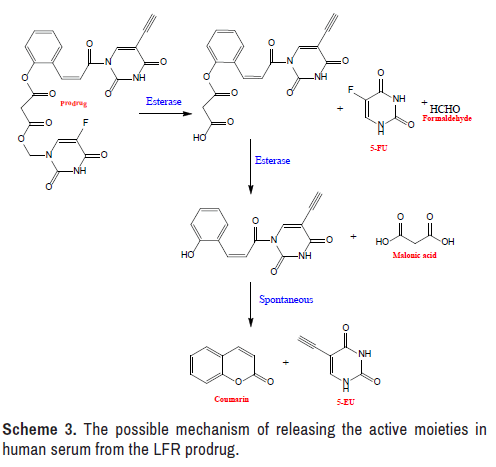
The hydrolysis of the produced LFR prodrug in human serum was monitored using an approach similar to that used to investigate chemical stability. The investigation was done by observing the rise in the concentration of 5-EU versus time, with the exceptions of replacing the buffer solution with serum and replacing the buffer solution with serum [44– 47]. Since 5-EU is the last product released from the mutual prodrug under the action of the esterase enzyme, as illustrated in the concentration of this agent was examined (Scheme 3).
Results and Discussion
Design of the LFR prodrug
The synthetic LFR prodrug was created in order to improve the therapeutic utility of FU as an oral drug. Three concerns were addressed in order to achieve this goal. The first is to choose a prodrug that increases FU's lipophilicity, reduces its degradation by DPDE, and allows for mutual effect. The log P values for the FU, EU, and target prodrug were determined to be -0.90, -0.51, and 1.76, respectively, in this regard. This suggests that the target LFR prodrug has a higher lipophilicity than its predecessor medications, which might help increase FU oral bioavailability. Furthermore, releasing FU and EU at the same time may minimize the degradation of FU by DPDE, allowing for mutual activity. The research of prodrug stability in medium with pH values that mimic those observed in the gastrointestinal system is the second topic. The final is the capacity of the synthesized LFR prodrug to release FU and EU simultaneously in a human serum with an appropriate half-life.
Synthetic pathway
The synthesis of the LFR prodrug involves a seven-step linear process, as illustrated in Scheme-2, and is a simple variant on the one described by Mustafa and Al-Omari [31]. This version used a malonyl linkage to bind the phenolic hydroxyl group of the carrier molecule to the phenolic hydroxyl group of chemical a. LiAlH4 was used to convert coumarin into an open ring diol under very strict circumstances. To avoid the reduction of the exocyclic double bond, the temperature was controlled below 0°C and the reaction duration was kept under 15 minutes, and the catalyst was of high purity to reduce side reactions. TBDMS-Cl was used to protect the allylic hydroxyl group as silyl ether in the second phase. The phenolic hydroxyl group then collaborated with the previously synthesized chemical as to produce a diester connection utilizing malonyl chloride as an anchor in the following step. In the fourth stage, an acid deprotected the allylic hydroxyl group, which was then oxidized into allylic aldehyde by a selective oxidizing agent, MnO2, in the next step. In the last synthesis stages, NaClO2 and H2O2 were used to oxidize the allylic aldehyde to allylic carboxylic acid, which was then linked with EU through DCC to yield the target LFR prodrug.
Structural characterization
The physicochemical parameters and spectral data for the target LFR prodrug and its reaction intermediates obtained from the used instruments are provided in the supplementary file. The chemical frameworks of the produced compounds were validated by these findings.
In vitro kinetic studies
Chemical stability: The LFR prodrug displayed significant chemical stability in the HCl buffer and phosphate-buffered saline under experimental circumstances, with half-lives of 33.19 hours and 18.13 hours, respectively. This stability may be due to the steric hindrance surrounding the ester bonds, which provides excellent resistance to nucleophilic attack [48]. This discovery also indicated that the prodrug can pass through the media intact with a pH range similar to that of the gastrointestinal system [49–51].
Despite the fact that the hydrolysis of the LFR prodrug in the used buffers is dependent on two parameters, including the prodrug and attacking agent concentrations, the kinetics was observed to be pseudofirst- order [52–54]. This is because the attacking agent's concentration is exceedingly high in relation to the prodrug's, causing its influence on the hydrolysis kinetics to be overlooked [55].
Release study
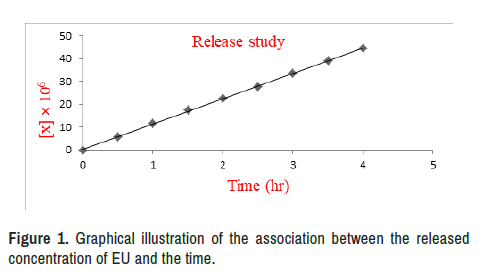
The prepared prodrug was capable of releasing the active two moieties in a zero-order kinetics manner, with a t1/2 of 4.62 hours (Tables 1-3). This discovery demonstrated that the LFR prodrug had a long enough circulation time to reach the target and free the two drugs [56]. The LFR prodrug can be administered orally in a low-frequency mode, based on the kinetics characteristic [57], as a result of which patient compliance has improved [58].
| Absorbance | Time(hr) | X (M × 106) | a-x (M × 106) | In a/a-x |
| 0.1328 | 0.0 | 0.0000 | 100.0000 | 0.0000 |
| 0.1316 | 0.5 | 0.9411 | 99.0589 | 0.0095 |
| 0.1303 | 1.0 | 1.8742 | 98.1258 | 0.0189 |
| 0.1288 | 1.5 | 3.0120 | 96.9880 | 0.0306 |
| 0.1282 | 2.0 | 3.4639 | 96.5361 | 0.0353 |
| 0.1267 | 2.5 | 4.6256 | 95.3744 | 0.0474 |
| 0.1258 | 3.0 | 5.2711 | 94.7289 | 0.0542 |
| 0.1240 | 3.5 | 6.6265 | 93.3735 | 0.0686 |
| 0.1231 | 4.0 | 7.2786 | 92.7214 | 0.0756 |
| Note. a= prodrug concentration at zero time that equals to 100 M, and (a-x)=Residual concentration of prodrug at defined time. | ||||
| Absorbance | Time(hr) | X (M × 106) | a-x (M × 106) | In a/a-x |
|---|---|---|---|---|
| 0.1301 | 0.0 | 0.0000 | 100.0000 | 0.0000 |
| 0.1280 | 0.5 | 1.6329 | 98.3671 | 0.0165 |
| 0.1259 | 1.0 | 3.2414 | 96.7586 | 0.0330 |
| 0.1240 | 1.5 | 4.6887 | 95.3113 | 0.0480 |
| 0.1215 | 2.0 | 6.6103 | 93.3897 | 0.0684 |
| 0.1198 | 2.5 | 7.9020 | 92.0980 | 0.0823 |
| 0.1177 | 3.0 | 9.5311 | 90.4689 | 0.1002 |
| 0.1162 | 3.5 | 10.6841 | 89.3159 | 0.1130 |
| 0.1140 | 4.0 | 12.3422 | 87.6578 | 0.1317 |
| Absorbance | Time (hr) | x (M × 106) |
|---|---|---|
| 0.0000 | 0.0 | 00.0000 |
| 0.0954 | 0.5 | 5.8080 |
| 0.0989 | 1.0 | 11.7207 |
| 0.1032 | 1.5 | 17.4477 |
| 0.1079 | 2.0 | 22.4918 |
| 0.1118 | 2.5 | 27.9017 |
| 0.1167 | 3.0 | 33.4394 |
| 0.1216 | 3.5 | 38.9771 |
| 0.1267 | 4.0 | 44.6184 |
Provide the results of the in vitro kinetic investigations, whereas Table 4 shows the calculated kinetic parameters depicted a graph illustrating the association between the released concentration of EU and the passage of time (Figure 1).
| HCI (pH 1.2) buffer | Phosphate- buffered saline (pH 6.8) | Serum |
|---|---|---|
| ɛ= 284 L mol-1 cm-1 | ɛ=298 L mo1-1 cm-1 | ɛ=1930.86 L mo1-1 |
| λ max=281 nm | λ max=312 mm | λ max =292 nm |
| t ½ =33.19 hr | t ½=18.13 hr | t ½=4.62 hr |
| koba=5.8 × 106 hr-1 | koba=10.62 × 106 hr-1 | koba=10.83 × 106 M.hr-1 |
| ɛ=Absorbance coefficient, and Koba=observed rate constant. | ||
Conclusion
Using an LFR prodrug system, this research found that FU and its powerful metabolic modulator, EU, may be combined into a single chemical entity. The synthesized mutual prodrug was stable in medium with pH values that mimicked those observed in the gastrointestinal system, according to in vitro kinetic tests. In addition, the LFR prodrug was able to release FU and EU in a human serum with a t1/2 of 4.62 hours, following zero-order kinetics. The t1/2 value enables the LFR prodrug to reach the target intact, resulting in an increase in therapeutic effectiveness.
Conflict of Interest
The authors stated that there is no conflict of interest.
References
- Cui, Qi N, Yen C Hsia, Shan C Lin and Robert L Stamper, et al. “Effect of Mitomycin c and 5-Flurouracil Adjuvant Therapy on the Outcomes of Ahmed Glaucoma Valve Implantation.” Clin Exp Ophthalmol 45 (2017): 128-134.
- DA, Som KIM, Kyoungmi Min, and Suk Kyeong Lee. “Cell Cycle Dysregulation Is Associated With 5-Fluorouracil Resistance in Gastric Cancer Cells.” Anticancer Res 40 (2020): 3247-3254.
- Blondy, Sabrina, Valentin David, Mireille Verdier and Muriel Mathonnet, et al. “5-Fluorouracil Resistance Mechanisms in Colorectal Cancer: From Classical Pathways to Promising Processes.” Cancer Sci 111 (2020): 3142.
- Zhu, Guangwei, Ming Zhao, Qinghong Han and Yuying Tan, et al. “Combination of Trabectedin with Oxaliplatinum and 5-Fluorouracil Arrests a Primary Colorectal Cancer in a Patient-Derived Orthotopic Xenograft Mouse Model.” Anticancer Res 39 (2019): 5999-6005.
- Nishizawa, Yukihiko, Ryuji Ikeda, Masatatsu Yamamoto and Kohichi Kawahara, et al. “5-aza-2-Deoxycytidine Enhances the Sensitivity of 5-Fluorouracil by Demethylation of the Thymidine Phosphorylase Promoter.” Anticancer Res 39 (2019): 4129-4136.
- Cavaliere, Alessandra, Katrin C Probst, Andrew D Westwell and Magdalena Slusarczyk. “Fluorinated Nucleosides as an Important Class of Anticancer and Antiviral Agents.” Future Med Chem 9 (2017): 1809-1833.
- Rivera, Edgardo, Jenny C Chang, Vladimir Semiglazov and Olga Burdaeva, et al. “Eniluracil Plus 5-Fluorouracil and Leucovorin: Treatment for Metastatic Breast Cancer Patients in Whom Capecitabine Treatment Rapidly Failed.” Clin Breast Cancer 14 (2014): 26-30.
- Mindt, Sonani, Sihem Aida, Kirsten Merx and Annette Müller, et al. “Therapeutic Drug Monitoring (TDM) of 5-Fluorouracil (5-FU): New Preanalytic Aspects.” Clin Chem Lab Med 57(2019): 1012-1016.
- Pasban, Samaneh, Heidar Raissi, Majid Pakdel, and Farzaneh Farzad. “Enhance the Efficiency of 5-Fluorouracil Targeted Delivery by Using a Prodrug Approach as a Novel Strategy for Prolonged Circulation Time and Improved Permeation.” Int J Pharm 568 (2019): 118491.
- Ota, Yosuke, Arisa Nakamura, Elghareeb E Elboray, Yukihiro Itoh and Takayoshi Suzuki. “Design, Synthesis, and Biological Evaluation of a Conjugate of 5-Fluorouracil and an LSD1 Inhibitor.” Chem Pharm Bull (2018): c18-00577.
- Giret, Simon, Christophe Théron, Audrey Gallud and Marie Maynadier, et al. “A Designed 5-Fluorouracil Based Bridged Silsesquioxane as an Autonomous Acid" Triggered Drug "Delivery System.” Chemistry 19 (2013): 12806-12814.
- Wen, Zhiwei, Paloma R Tuttle, A Hasan Howlader and Anna Vasilyeva, et al. “Fluorescent 5-Pyrimidine and 8-Purine Nucleosides Modified with an N-Unsubstituted 1, 2, 3-Triazol-4-yl Moiety.” J Organic Chem 84 (2019): 3624-3631.
- Oglah, Mahmood Khudhayer, and Yasser Fakri Mustafa. “Curcumin Analogs: Synthesis and Biological Activities.” Med Chem Res 29 (2020): 479-486.
- Oglah, Mahmood Khudhayer, Yasser Fakri Mustafa, Moath Kahtan Bashir and Mahmood Hashim et al. “Curcumin and its Derivatives: A Review of their Biological Activities.” Syst Rev Pharm 11 (2020): 472.
- Oglah, Mahmood Khudhayer and Yasser Fakri Mustafa. “Synthesis, Antioxidant, and Preliminary Antitumor Activities of New Curcumin Analogues.” J Glob Pharma Technol 12 (2020): 854.
- Bashir, Moath Kahtan, Yasser Fakri Mustafa and Mahmood Khudhayer Oglah. "Antitumor, Antioxidant, and Antibacterial Activities of Glycosylconjugated Compounds: A Review.” Syst Rev Pharm 11 (2020): 175.
- Mustafa, Yasser Fakri, Moath Kahtan Bashir and Mahmood Khudhayer Oglah. “Original and Innovative Advances in the Synthetic Schemes of Coumarin-Based Derivatives: A Review.” Sys Rev Pharm 11 (2020): 598-612.
- Slavíaková, Michaela, Martina Janoušková, Anna Šimonová and Hana Cahová, et al. “Turning Off Transcription with Bacterial RNA Polymerase through CuAAC Click Reactions of DNA Containing "Ethynyluracil.” Chemistry 24 (2018): 8311-8314.
- Kartsev, Victor, Athina Geronikaki, Silvia Bua and Alessio Nocentini, et al. “Extending the Inhibition Profiles of Coumarin-Based Compounds Against Human Carbonic Anhydrases: Synthesis, Biological, and in Silico Evaluation.” Molecules 24 (2019): 3580.
- Mustafa, Yasser Fakri, Raghad Riyadh Khalil and Eman Tareq Mohammed. “Antimicrobial Activity of Aqueous Extracts Acquired from the Seeds of Two Apples’ Cultivars.” Sys Rev Pharm 11(2020): 382-387.
- Mustafa, Yasser Fakri, Eman Tareq Mohammed and Raghad Riyadh Khalil. “Antioxidant and Antitumor Activities of Methanolic Extracts Obtained from Red Delicious and Granny Smith Apples' Seeds.” Sys Rev Pharm 11 (2020): 570-576.
- Mohammed, Eman Tareq and Yasser Fakri Mustafa. “Coumarins from Red Delicious Apple Seeds: Extraction, Phytochemical Analysis, and Evaluation as Antimicrobial Agents.” Sys Rev Pharm 11 (2020): 64-70.
- Khalil, Raghad Riyadh and Yasser Fakri Mustafa. “Phytochemical, Antioxidant and Antitumor Studies of Coumarins Extracted from Granny Smith Apple Seeds by Different Methods.” Syst Rev Pharm 11(2020): 57-63.
- Xie, Qiong, Xiaolin Wang, Xinghai Wang, Zhiqiang Jiang and Zhuibai Qiu. “Design, Synthesis, and Bioavailability Evaluation of Coumarin-Based Prodrug of Meptazinol.” Bioorg Med Chem Lett 15 (2005): 4953-4956.
- Wang, Wei, Gian Camenisch, David C Sane, Huijuan Zhang and Erin Hugger, et al. “A Coumarin-Based Prodrug Strategy to Improve the Oral Absorption of RGD Peptidomimetics.” J Control Release 65 (2000): 245-251.
- Wang, Binghe, Huijuan Zhang, Ailian Zheng and Wei Wang. “Coumarin-Based Prodrugs. Part 3: Structural Effects on the Release Kinetics of Esterase-Sensitive Prodrugs of Amines.” Bioorg Med Chem 6 (1998): 417-426.
- Yang, Xinying, Xuben Hou, Binghe Wang and Minyong Li, et al. “Density Functional Theory Based Quantitative Structure-Property Relationship Studies on Coumarin-Based Prodrugs.” Bio Sci Trends 6 (2012): 234-240.
- Aspatwar, Ashok, Emanuela Berrino, Silvia Bua and Fabrizio Carta, et al. “Toxicity Evaluation of Sulfamides and Coumarins that Efficiently Inhibit Human Carbonic Anhydrases.” J Enzyme Inhib Med Chem 35 (2020): 1765-1772.
- Mustafa, Yasser Fakri, Mahmood Khudhayer Oglah and Moath Kahtan Bashir. “Conjugation of Sinapic Acid Analogues with 5-Fluorouracil: Synthesis, Preliminary Cytotoxicity, and Release Study.” Syst Rev Pharm 11 (2020): 482.
- Nejres, Aws Maseer, Hameed Khalid Ali, Safaa Polus Behnam and Yasser Fakri Mustafa. “Potential Effect of Ammonium Chloride on the Optical Physical Properties of Polyvinyl Alcohol.” Sys Rev Pharm 11(2020): 726-732.
- Mustafa, Yasser F and Nohad A Al-Omari. “Design, Synthesis and Kinetic Study of Coumarin-Based Mutual Prodrug of 5-Fluorouracil and Dichloroacetic Acid.” Iraq J Pharm Sci 25 (2016): 6-16.
- Nejres, Aws M, Yasser Fakri Mustafa and Hasan S Aldewachi. “Evaluation of Natural Asphalt Properties Treated with Egg Shell Waste and Low Density Polyethylene.” Int J Pavement Eng (2020): 1-7.
- Bashir, Moath Khtan, Yasser Fakri Mustafa and Mahmood Khudhayer Oglah. “Synthesis and Antitumor Activity of New Multifunctional Coumarins.” Period Tche Quim 17 (2020): 871-883.
- Mustafa, Yasser Fakri. “Synthesis Characterization and Antibacterial Activity of Novel Heterocycle, Coumacine, and Two of its Derivatives.” Saudi Pharm J 26 (2018): 870-875.
- Mustafa, Yasser Fakri. “Synthesis Characterization and Preliminary Cytotoxic Study of Sinapic Acid and its Analogues.” J Glob Pharma Technol 11 (2019): 1-10.
- Mustafa, Yasser Fakri, Moath Abdulla Najem and Zena Sideek Tawffiq. “Coumarins from Creston Apple Seeds: Isolation, Chemical Modification, and Cytotoxicity Study.” J Appl Pharm Sci 8 (2018): 049-56.
- Mahmood, AA, YS Mustafa, and M Abdulstaar. “New Coumarinic Azo-Derivatives of Metoclopramide and Diphenhydramine: Synthesis and in Vitro Testing for Cholinesterase Inhibitory Effect and Protection Ability Against Chlorpyrifos.” Med J Malaysia 13 (2014): 1-2.
- Mustafa, Yasser Fakri, Mahmood Khudhayer Oglah, Moath Kahtan Bashir and Eman Tareq Mohammed, et al. “Prodrug of 5-Fluorouracil and 5-Ethynyluracil: Synthesis, Characterization, and Release Study.” Annals Roman Soc Cell Bio (2021): 5671-5688.
- Hwang, Nicky, Yonggang Pei, Jason Clement and Erle S. Robertson, et al. “Identification of a 3-β-Homoalanine Conjugate of Brusatol with Reduced Toxicity in Mice.” Bioorg Med Chem Lett 30 (2020): 127553.
- Aldewachi, Hasan, Yasser Fakri Mustafa, Rahma Najm and Farah Ammar. “Adulteration of Slimming Products and its Detection Methods.” Syst Rev Pharm 11 (2020): 289.
- Man, Gene Chi Wai, Jianzhang Wang, Yi Song and Jack Ho Wong, et al. “Therapeutic Potential of a Novel Prodrug of Green Tea Extract in Induction of Apoptosis via ERK/JNK and Akt Signaling Pathway in Human Endometrial Cancer.” BMC Cancer 20 (2020): 1-14.
- Oglah, Mahmood Khudhayer, Moath Kahtan Bashir, Yasser Fakri Mustafa and Eman Tareq, et al. “Synthesis and Biological Activities of 3,5-Disubstituted-4-Hydroxycinnamic Acids Linked to a Functionalized Coumarin.” Sys Rev Pharm 11(2020): 717-725.
- Mustafa, Yasser Fakri, Noora Thamer Abdulaziz, Raghad Riyadh Khalil and Eman Tareq Mohammed, et al. “A Review on the Folate-Linked Prodrugs for Cancer Chemotherapy.” Annals Roman Soc Cell Bio (2021): 5645-5670.
- Watanabe, Shimpei, Svante Vikingsson, Markus Roman and Henrik Green, et al. “In Vitro and in Vivo Metabolite Identification Studies for the New Synthetic Opioids Acetylfentanyl, Acrylfentanyl, Furanyl fentanyl, and 4-Fluoro-Isobutyrylfentany.” AAPS J 19 (2017): 1102-1122.
- Mustafa, Yasser Fakri, and Noora Thamer Abdulaziz. “Biological Potentials of Hymecromone-Based Derivatives: A Systematic Review.” Syst Rev Pharm 1 (2020): 438-452.
- Mustafa, Yasser Fakri and Noora Thamer Abdulaziz. “4-Methylumbelliferone and its Derived Compounds: A Brief Review of Their Cytotoxicity.” Egyptian J Chem 64 (2021): 1807-1876.
- Mustafa, Yasser Fakri and Noora Thamer Abdulaziz. “A Review on the Antineoplastic Activity of Hymecromone and its Based Products.” Anna Roman Soc Cell Bio 25 (2021): 13339-13354.
- Li, Man, Zhen Liang, Xun Sun, and Tao Gong, et al. “A Polymeric Prodrug of 5-Fluorouracil-1-Acetic Acid Using a Multi-Hydroxyl Polyethylene Glycol Derivative as the Drug Carrier.” PLoS One 9 (2014): e112888.
- Zhao, Dongyang, Huicong Zhang, Wenhui Tao and Wei Wei, et al. “A Rapid Albumin-Binding 5-Fluorouracil Prodrug with a Prolonged Circulation Time and Enhanced Antitumor Activity.” Biomater Sci 5 (2017): 502-510.
- Bashir, Moath Kahtan, Mahmood Khudhayer Oglah and Yasser Fakri Mustafa. “The Effect of Aryl and Heteroaryl Conjugation on the Biological Activities of Naphthalenes: A Review.” Annals Roman Soc Cell Bio (2021): 13355-13379.
- Mustafa, Yasser Fakri and Noora Thamer Abdulaziz. “Hymecromone and Its Derivatives as Promising Cytotoxic Agents: A Review.” Annals Roman Soc Cell Bio (2021): 6974-6981.
- Stroberg, Wylie and Santiago Schnell. “On the Validity and Errors of the Pseudo-First-Order Kinetics in Ligand–Receptor Binding.” Math Biosci 287 (2017): 3-11.
- Mustafa, Yasser Fakri. “Synthesis, Characterization, and Biomedical Assessment of Novel Bisimidazole–Coumarin Conjugates.” Applied Nanosci (2021): 1-12.
- Mustafa, Yasser Fakri, SM Kasim, BM Al Dabbagh and Al Shakarchi. “Synthesis, Characterization and Biological Evaluation of New Benzimidazoles.” Appl Nanosci 52 (2021): 1-2.
- Anzo, Kenji, Makoto Harada and Tetsuo Okada. “Enhanced Kinetics of Pseudo First-Order Hydrolysis in Liquid Phase Coexistent with Ice.” J Phys Chem A 117 (2013): 10619-10625.
- Zhang, Lu, Joy Alfano, Doran Race and Rajesh N Davé. “Zero-Order Release of Poorly Water-Soluble Drug from Polymeric Films Made via Aqueous Slurry Casting.” Eur J Pharm Sci 117 (2018): 245-254.
- M Laracuente, MH Yu and KJ Mchugh. “Zero-Order Drug Delivery: State of the Art and Future Prospects.” J Control Release 327 (2020): 834-856.
- Wen, Na, Yansheng Dong, Rui Song, Wenpeng Zhang and Chao Sun, et al. “Zero-Order Release of Gossypol Improves its Antifertility Effect and Reduces its Side Effects Simultaneously.” Biomacromolecules 19 (2018): 1918-1925.
Citation: Mustafa, Yasser Fakri, Mahmood Khudhayer Oglah, Moath Kahtan Bashir and Eman Tareq Mohammed, et al. "Mutual Prodrug of 5-Ethynyluracil And 5-Fluorouracil: Synthesis and Pharmacokinetic Profile" Clin Schizophr Relat Psychoses 15 (2021). Doi:10.3371/CSRP. MYMO.082521.
Copyright: © 2021 Mustafa YF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.