Research - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Tamara Amer Taha, Department of Science, University of Diyala, Baqubah, Iraq, Email: drtamaraamertaha@gmail.com
Received: 04-Aug-2021 Accepted Date: Aug 18, 2021 ; Published: 25-Aug-2021
Abstract
Over 200 of different types of Human Papilloma Virus (HPV) have been described high oncogenic HPV16 and HPV18 also have been related to subsets of Head and Neck Squamous Cell Carcinoma (HNSCC) and laryngeal lesions. It's been reported that elevated levels of E6 and E7 expressions of hrHPV genotypes as well as tumor suppressor genes expression might have a role in pathogenesis and tumor genesis. The aim of this study is to detect the nuclear co-localization of high risk oncogenes HPV16 and HPV18(E6)/(E7)proteins expressions with over expression tumor suppressor gene Rb protein in patients with different types of laryngeal lesions. A retrospective study hypothesis, one hundred and 57 Formalin-Fixed, Paraffin-Embedded (FFPE) specimens were collected. Molecular detection and genotyping of high risk HPV 16 and 18 genotypes were conducted by Chromogenic Insituhybridization (CISH).The results of present study showed the highest mean value of age was in laryngeal malignant groups (56.91 ± 17.12), while the lowest mean age was at control groups (27.25 ± 12.15). The mean age of male (43.43 ± 19.91) was more than the mean age of female (39.68 ± 19.25).The present study results and according to high risk oncogenes (E6) and (E7) expression of HPV16 and HPV18 as well as Rb expression suggest that high oncogenic HPV pointed that high oncogenic HPV genotypes could have a possible role in laryngeal pathogenesis and early steps of benign and malignant tumor genesis as well as carcinogenesis of larynx.
Keywords
Tumor • Oncogenic • Carcinogenesis
Introduction
Over 200 of different types of Human Papilloma Virus (HPV) has been described [1]. Human papilloma virus can infect epidermal or mucosal epithelial cells and cause many benign lesions and may cause persistence, malignant transformation to caner. HPV persistent infection may base on several characteristics, like HPV types and variants, raised viral loads and peoples cellular responses to HPV infection, however, most HPV infection resolve spontaneously [2,3]. According to risk of malignancy; HPV has been grouped to elevate risk HPV and lowed risk HPV high risk HPV genotypes such as HPV16, HPV18, HPV31, HPV33, HPV35 and HPV45 can cause malicious progression of lesions in many human tissues organs [4]. Depending on epidemiological and molecular studies, the association between the infection with high risk HPV genotypes and genital tumors has been establish since 1970s.HPV16 and HPV18 also have been related with subsets of Head and Neck Squamous Cell Carcinoma (HNSCC)as well as lesions such as laryngeal lesions also HPV DNA have been detected in benign lesions (nodules, polyps, papillomatosis and verrucous carcinoma [5-7]. The presence of HPV genotypes in laryngeal malignant and benign lesions especially high oncogenic genotypes as HPV16 and HPV18 has been detected in about 0%-76.42% [8]. The HPV DNA have the ability to integrate itself on to host cell genome and use by using its transcription and translation machinery in order to express viral proteins from many early genes lead to cell metabolic disorder, genetic mutation, expression genes products disorder and malignant proliferation of host cells [9]. High Risk (HR) HPV E6 and E7 has an oncogenic potential to cripple cell cycle check points result in inhibit tumor suppressor genes (P53 and P Rb) respectively leading to elevate levels of E6 and E7 enhancing cell transformation and immortalization through many cellular events induce premalignant HPVassociated squamous intra– epithelial lesions [10,11]. The E7 protein of HPV16 and HPV18 interact and bind to Retinoblastoma (RB) family of tumor suppressor proteins (pRb) and damage RB/E2F complex through ubiquitin pathway, resulting in cell division [12]. Rb proteins are a product of Rb gene has a fundamental role in the regulation of growth cell cycle. It is a phosphoprotein of 105-110 KDa and its locus on chromosome 13q14. Rb is a member of pocket protein family (also include; p107 and p130) whose members have a functional binding of other proteins. It has been documented that the expression of retinoblastoma (pRb) when analyzed may give a powered parameter of a connection of causation between HPV infection and HNSCC [13]. Decreased or absence pRb expression was presented to be importantly associated with the appearance of HPV DNA [14].
Most of laryngeal cancer is squamous cell carcinoma and it has the second highest incidence of the upper aero digestive tract malignancies around the word [15]. Laryngeal Squamous Cell Carcinoma (LSCC) constitutes 3% of all adult cancers. In last years, it has been documented that LSCC has grown steadily, threating human health and life [16]. Despite the traditional conditions that result in laryngeal lesions as smoking, drinking, a differences sex hormone levels, air pollution and viral oncogenic infections such as infection with HPV genotypes especially high oncogenic one [17]. Epidemiologically, studies have been reported the rate of HPV infection in laryngeal cancer varying from 0%-79%. About 28% of HPV infection documented in all LSCC up to our knowledge, the current study represents the first in Iraq that highlighting for a possible role of HPV infection in Iraqi patients with laryngeal lesion and to elucidate the relationship exist between HPV infection and laryngeal benign and malignant lesions through evaluate the interaction and expression of high HPV oncogenes (E6,E7) and tumor suppressor gene PRb [18].
Materials and Methods
The study was retrospective one a total number of one hundred and 57 of formalin fixed ,paraffin embedded blokes of different laryngeal tissues samples that related to different laryngeal lesions from patients who had been exposed to resection or endoscopic laryngeal biopsy divided onto: Forty five (45) sample of laryngeal tissue cancers,72 benign laryngeal tissue samples divided into: Thirty five (35) laryngeal polyps and thirty seven (37) laryngeal nodules and forty (40) other laryngeal autopsies blocks sample were collected and labeled as apparently healthy control (laryngeal tissue samples without any significant histopathalogical changes after microscopic examination). The range of patients' laryngeal tissue specimen’s ages was 2-83 years. All of laryngeal tissue blocks samples are collected from archive of histopathalogical laboratory of AL-Hariri hospital Baghdad, AL-Yarmok teaching hospital, AL-Kindy teaching hospital and Baquba teaching hospital as well as from many private laboratories. All samples assembled during the period from January 2014 till February 2016. The diagnosis was depending on the recoded histopathalogical documents of the corresponding laryngeal lesions blocks after confirmatory histopathalogical re-examination by histopathologist for each obtained tissue blokes. All tissues blocks were subjected to cut as serial thin sections of 4 μm thickness. All tissues were stocked on charged slides except one for hematoxylin and eosin examinations for each laryngeal tissues lesions and apparently healthy control tissues.
Molecular assessment and genotyping of human papilloma virus DNA in laryngeal tissue blocks were carried out by advanced and recent generation of Chromomeric In Situ Hybridization (CISH) (Zytovision GmbH, Germany)were utilized to targeted DNA sequences within tissue samples using a cocktail of Digoxigenin-labeled long DNA probes (T-1144-400 ,ZytoVision GmbH ,Bremerhaven, Germany)for screening HPV genotypes (6,11,16,18,31,33,35,39,45,51,52,56,58,59,66,68,82),whereas genotyping of HPV was done by using a specific Digoxigenin -labeled HPV DNA probes. The (CISH) assay protocol used in this investigation was carried out according to the manufacturer's instructions. All chemicals were supplied except the diluted probe for the positive reactions, which were accomplished by substituting the probe with a Digoxigenin in house kipping gene probe.
The data was analyzed under light microscopy employing (100x, 400x, and 1000x) for identifying positive cells): (CISH) was assigned a brightness and percentage score depending on the number of positive signals and the frequency of signals, accordingly. Immune histochemical method was carried out to detect the prevalence PRb over expression protein in different laryngeal tissue lesions and was done according to the manufacturing company of antibody 80436-Expose Mouse and Rabbit specific HRP/ DAB Detection Kit (Abcam). This kit was used for detection of anti-pRbAb [PAb240] ab 26, Anti HPV 16E6 Antibody (CIP5), ANTI-HPV16(E7) antibody (SPM405)and Anti-HPV18 E7antibody (8E2) ab 100953 in laryngeal lesions were done according to the manufacturing company (Abcam, UK). P Rb was evaluated in laryngeal tissues as an intensity of nuclear and cytoplasmic stain and the positive cells percentage. No staining and severe staining were used to evaluate the strength of cytoplasmic and nuclear staining.
Statistical analysis
The Chi-square (X2) test was used to analyze percentages in the existing study's data. (Mean SD) was used to denote numeric dates. The T test was used to analyze two numeric variables, while the F test has been used to compare multiple numeric variables. A significance threshold of 0.05 was used to the test. Current data was analyzed using (SPSS v.22 and Graph pad prism v.6) applications.
Results
This study designed as a retrospective one. The role of human papilloma virus in laryngeal lesions was tested markers in histopathalogical specimens to risk of malignancy is observing significantly in higher (or lower) tendency for laryngeal lesions tissues from malignant cancer to benign lesions tissues (nodules and polyp)to express a positive markers in comparison to apparently healthy laryngeal tissues. The magnitude of expression of each test marker was measured in two different ways; first: Intensity measured with ordinal categories (strong stained and no stained). The second system is a scoring with ordinal categories ranging from 0-4 for positive stain (negative, low (+1), moderate (+2), high (+3) at high power field examination constitution).
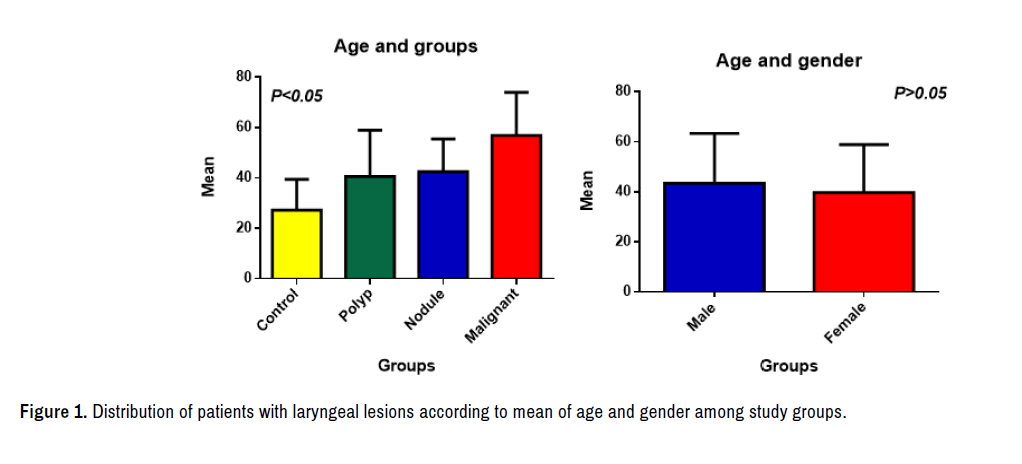
Distribution of age and sex among study groups
Based on the relation between age and groups, results of our study showed the age range of all study groups was (2-83) years, the highest mean value of age in malignant laryngeal lesion tissues groups was (56.91 ± 17.12) with age range from (8-83) years, while the lowest mean value was at control groups (27.25 ± 12.15) years old with the mean age range (2-66) years. the statistical analysis showed highly significant differences among groups according to mean age (p<0.05).While depending on age and gender as in Figure 1.The results of present study showed the mean of male (43.43 ± 19.91) was higher than the mean of female (39.68 ± 19.25) with no significant different (p>0.05).
HrHPV16+18(E6)/PRb-IHC
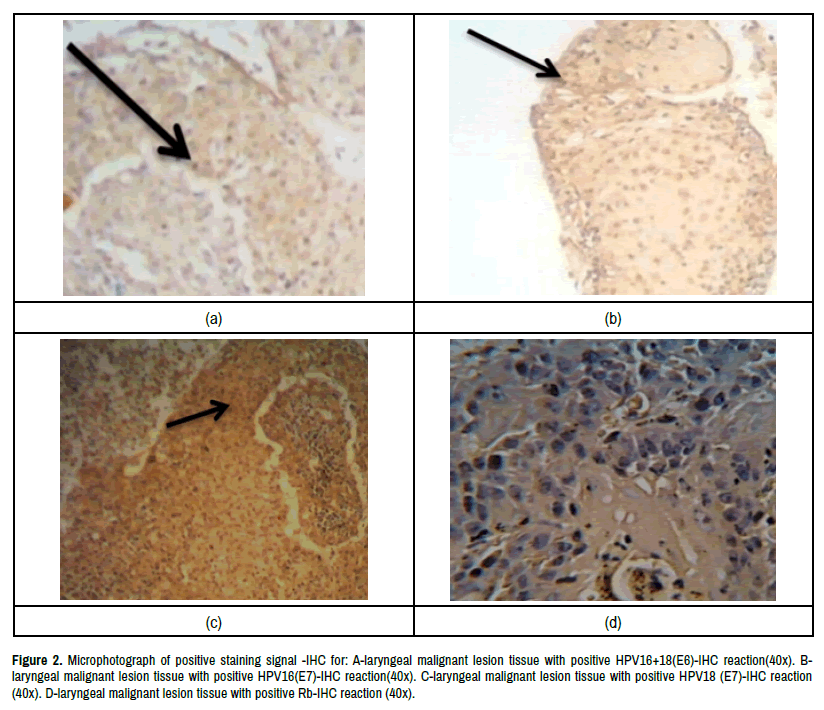
HrHPV16+18(E6) /PRB-IHC intensities: The percentage of HPVinfected cells was evaluated by intensity of early protein HPV16+18(E6) – IHC reaction signals intensities in relation to pRb-IHC intensities at a high power fields in laryngeal lesions, tissues under study. In malignant laryngeal lesion tissues group.The strong straining signal intensities of hrHPV16+18 (E6) protein expression -IHC in relation to pRb expression-IHC intensities were founded in (50.0%:4), (35.1%:13) respectively while no staining signal intensities of hrHPV16+18(E6) /PRb were detected in (64.9%:24). In polyps laryngeal lesion tissues group. The Positive strong staining signal intensities ofhrHPV16+18 (E6)/ PRb-IHC were observed in (0.0%:0), (9.7%:3) respectively, while no staining signal intensities of hrHPV16+18 (E6)/PRb-IHC over expression was detected in (90.3%:28). In nodules laryngeal lesion tissues group, the strong signal intensities were observed in (0.0%:0) and (3.1%:1) respectively while no staining signal was observed in (96.9%:31) whereas the positive strong signal of hrHPV16+18(E6)/PRb- IH Cover expression intensities were founded in (0.0%:0) and (8.1%:3 hrHPV16+18E6(+)/PRb(-) no staining signal intensities were observed in (91.9%:34). Statistically, there are no significant differences among groups except nodules tumors group as well as apparently healthy control (p<0.05) as in Table 1 and Figure 2A.
| Studied groups | Rb intensity | X2 (P-value) |
||||
|---|---|---|---|---|---|---|
| NO stain | Strong | |||||
| A.H. Control |
HPV16+18(E6) intensity |
NO stain | N | 34 | 3 | P=0.608 Non Sign. (P>0.05) |
| % | 91.9% | 100.0% | ||||
| Strong | N | 3 | 0 | |||
| % | 8.1% | 0.0% | ||||
| Total | N | 37 | 3 | |||
| % | 100.0% | 100.0% | ||||
| Nodules Benign laryngeal lesion tissues |
HPV16+18(E6) intensity |
NO stain | N | 31 | 5 | P=0.689 Non Sign. (P>0.05) |
| % | 96.9% | 100.0% | ||||
| Strong | N | 1 | 0 | |||
| % | 3.1% | 0.0% | ||||
| Total | N | 32 | 5 | |||
| % | 100.0% | 100.0% | ||||
| Polyps Benign laryngeal lesion tissues |
HPV16+18(E6) intensity |
NO stain | N | 28 | 4 | P=0.515 Non Sign. (P>0.05) |
| % | 90.3% | 100.0% | ||||
| Strong | N | 3 | 0 | |||
| % | 9.7% | 0.0% | ||||
| Total | N | 31 | 4 | |||
| % | 100.0% | 100.0% | ||||
| Malignant laryngeal lesion tissues | HPV16+18(E6) intensity |
NO stain | N | 24 | 4 | P=0.432 Non Sign. (P>0.05) |
| % | 64.9% | 50.0% | ||||
| Strong | N | 13 | 4 | |||
| % | 35.1% | 50.0% | ||||
| Total | N | 37 | 8 | |||
| % | 100.0% | 100.0% | ||||
Figure 2.Microphotograph of positive staining signal -IHC for: A-laryngeal malignant lesion tissue with positive HPV16+18(E6)-IHC reaction(40x). Blaryngeal
malignant lesion tissue with positive HPV16(E7)-IHC reaction(40x). C-laryngeal malignant lesion tissue with positive HPV18 (E7)-IHC reaction
(40x). D-laryngeal malignant lesion tissue with positive Rb-IHC reaction (40x).
b-HrHPV16+18(E6)/PRB-IHC scores
The positive staining signal scores results of hrHPV16+18(E6)/PRb- IHC over expression in HPV-infected cells of Malignant laryngeallesion tissues group were constitute in (50.0% :4)with score(+) while (27.0%:10) and (8.1%:3) with score(+) and (++)respectively. The negative signal stain of hrHPV16+18(E6)/PRb-IHC was(64.9%:24). In polyps laryngeal lesion tissues group , the percentages of positive hrHPV16+18(E6)/pRb-IHC showed Low(+) and moderate (++) signal scores were detected in (0.0%:0) for each score whereas, the negative signal stain of hrHPV16+18(E6)/PRb- IHC for this group founded in (90.3%:28).In nodules laryngeal lesion tissues group, the percentages of positive signal score hrHPV16+18(E6)/PRb-IHC showed Low (+) signal scores were detected in (0.0%:0) while negative stain signal scores of HPV 16+18(E6) /PRb-IHC were(96.9%:31). In this group. In apparently healthy control, the percentage of negative signal score was (91.9%:34) while positive signal score was not observed in low score(+) and score (++), the percentage was (0.0%:0) each other. Importantly, there are no significant different among study groups as in Table 2.
| Studied groups | Rb scores | X2 (P-value) |
||||
|---|---|---|---|---|---|---|
| Negative | + | |||||
| A.H. Control |
HPV16+18 (E6) scores |
Negative | N | 34 | 3 | P=0.877 Non Sign. (P>0.05) |
| % | 91.9% | 100.0% | ||||
| + | N | 1 | 0 | |||
| % | 2.7% | 0.0% | ||||
| ++ | N | 2 | 0 | |||
| % | 5.4% | 0.0% | ||||
| Total | N | 37 | 3 | |||
| % | 100.0% | 100.0% | ||||
| Nodules Benign laryngeal lesion tissues |
HPV16+18 (E6) scores |
Negative | N | 31 | 5 | P=0.689 Non Sign. (P>0.05) |
| % | 96.9% | 100.0% | ||||
| + | N | 1 | 0 | |||
| % | 3.1% | 0.0% | ||||
| Total | N | 32 | 5 | |||
| % | 100.0% | 100.0% | ||||
| Polyps Benign laryngeal lesion tissues |
HPV16+18 (E6) scores |
Negative | N | 28 | 4 | P=0.809 Non Sign. (P>0.05) |
| % | 90.3% | 100.0% | ||||
| + | N | 2 | 0 | |||
| % | 6.5% | 0.0% | ||||
| ++ | N | 1 | 0 | |||
| % | 3.2% | 0.0% | ||||
| Total | N | 31 | 4 | |||
| % | 100.0% | 100.0% | ||||
| Malignant laryngeal lesion tissues | HPV16+18 (E6) scores |
Negative | N | 24 | 4 | P=0.368 Non Sign. (P>0.05) |
| % | 64.9% | 50.0% | ||||
| + | N | 10 | 4 | |||
| % | 27.0% | 50.0% | ||||
| ++ | N | 3 | 0 | |||
| % | 8.1% | 0.0% | ||||
| Total | N | 37 | 8 | |||
| % | 100.0% | 100.0% | ||||
HrHPV16 (E7)/PRb-IHC
HrHPV16 (E7)/PRb-IHC intensities: In malignant laryngeal lesion group, the positive strong staining signal intensities of hrHPV16(E7)/PRb- IHC were observed in (50.0%:4) while no staining signal intensities were found in (48.6%:18). In Polyp laryngeal lesions group, the strong staining signal intensities of hrHPV16(E7)/PRb-IHC over expression were observed in (25.0%:1) while no staining signal were founded in (87.1%:27). In nodules laryngeal lesions group, there wasn’t positive strong staining signal intensities were observed (0.0%:0) while no staining signal intensities were founded in (96.9%:31) of this group. Whereas in apparently healthy control group, there wasn’t positive strong staining signal intensities were observed ( 0.0%:0) while no staining signal intensities (94.6%:35) of hrHPV16(E7) /PRb-IHC expression. Statically, there weren’t significant differences in groups and among them(P<0.05) as in Table 3 and Figure 2B.
| Studied groups | Rb intensity | X2 (P-value) |
||||
|---|---|---|---|---|---|---|
| NO stain | Strong | |||||
| A.H. Control |
HPV16 E7 intensity |
NO stain | N | 35 | 3 | P=0.679 Non Sign. (P>0.05) |
| % | 94.6% | 100.0% | ||||
| Strong | N | 2 | 0 | |||
| % | 5.4% | 0.0% | ||||
| Total | N | 37 | 3 | |||
| % | 100.0% | 100.0% | ||||
| Nodules Benign laryngeal lesion tissues |
HPV16 E7 intensity |
NO stain | N | 31 | 5 | P=0.689 Non Sign. (P>0.05) |
| % | 96.9% | 100.0% | ||||
| Strong | N | 1 | 0 | |||
| % | 3.1% | 0.0% | ||||
| Total | N | 32 | 5 | |||
| % | 100.0% | 100.0% | ||||
| Polyps Benign laryngeal lesion tissues |
HPV16 E7 intensity |
NO stain | N | 27 | 3 | P=0.515 Non Sign. (P>0.05) |
| % | 87.1% | 75.0% | ||||
| Strong | N | 4 | 1 | |||
| % | 12.9% | 25.0% | ||||
| Total | N | 31 | 4 | |||
| % | 100.0% | 100.0% | ||||
| Malignant laryngeal lesion tissues |
HPV16 E7 intensity |
NO stain | N | 18 | 4 | P=0.945 Non Sign. (P>0.05) |
| % | 48.6% | 50.0% | ||||
| Strong | N | 19 | 4 | |||
| % | 51.4% | 50.0% | ||||
| Total | N | 37 | 8 | |||
| % | 100.0% | 100.0% | ||||
b.HrHPV16 (E7) /PRB-IHC scores
In malignant laryngeal lesions tissues group the positive HPV-infected cells with staining signal scores of hr HPV16(E7)-IHC in relation to PRb over expression-IHC were detected in moderate score(++) (50.0%:4 hrHPV16(E7)++/PRb+) and (35.1%:13hrHPV16(E7)++/PRb-) respectively, while the negative signal score were (48.6%:18). In polyps laryngeal lesions tissues group, the percentage of positive hrHPV16 (E7)/PRb-IHC show low (+) staining signal score in (25.0%:1), While negative signal score for this group was recorded in (87.1%:27). Those with laryngeal nodules tissues that hasn’t positive signal scores of hrHPV16 (E7)/PRb-IHC were constituted (0.0%:0) in moderate score (++) and (3.1%:1 hrHPV16 (E7)++/ PRb(-)-IHC , while the percentage those with negative signal score for this marker was (96.9%:31). Lastly, in apparently healthy control group, there weren’t positive signal (0.0:0) of hrHPV16(E7)/PRbin score (+) and (++) respectively, while (94.6%:35) has negative signal score. Statistically all groups haven’t significantly different (P>0.05) as in Table 4.
| Studied groups | Rb scores | X2 (P-value) |
||||
|---|---|---|---|---|---|---|
| Negative | + | |||||
| A.H. Control |
HPV16 E7 scores |
Negative | N | 35 | 3 | P=0.918 Non Sign. (P>0.05) |
| % | 94.6% | 100.0% | ||||
| + | N | 1 | 0 | |||
| % | 2.7% | 0.0% | ||||
| ++ | N | 1 | 0 | |||
| % | 2.7% | 0.0% | ||||
| Total | N | 37 | 3 | |||
| % | 100.0% | 100.0% | ||||
| Nodules Benign laryngeal lesion tissues |
HPV16 E7 scores |
Negative | N | 31 | 5 | P=0.689 Non Sign. (P>0.05) |
| % | 96.9% | 100.0% | ||||
| ++ | N | 1 | 0 | |||
| % | 3.1% | 0.0% | ||||
| Total | N | 32 | 5 | |||
| % | 100.0% | 100.0% | ||||
| Polyps Benign laryngeal lesion tissues |
HPV16 E7 scores |
Negative | N | 27 | 3 | P=0.632 Non Sign. (P>0.05) |
| % | 87.1% | 75.0% | ||||
| + | N | 3 | 1 | |||
| % | 9.7% | 25.0% | ||||
| ++ | N | 1 | 0 | |||
| % | 3.2% | 0.0% | ||||
| Total | N | 31 | 4 | |||
| % | 100.0% | 100.0% | ||||
| Malignant laryngeal lesion tissues |
HPV16 E7 scores |
Negative | N | 18 | 4 | P=0.431 Non Sign. (P>0.05) |
| % | 48.6% | 50.0% | ||||
| + | N | 6 | 0 | |||
| % | 16.2% | 0.0% | ||||
| ++ | N | 13 | 4 | |||
| % | 35.1% | 50.0% | ||||
| Total | N | 37 | 8 | |||
| % | 100.0% | 100.0% | ||||
HrHPV18 (E7)/PRB-IHC
HrHPV18 (E7)/PRB-IHC intensities: The percentages of hrHPVinfected cells with strong intensities of hrHPV18 (E7)/PRb over expression- IHC in (75.0%:6) while no stain signal intensities for this markers were founded in (70.3%: 26) in malignant laryngeal lesions tissues group. In laryngeal polyps lesions tissues group, the percentage of strong staining intensities of hrHPV18(E7)/PRb-IHC was observed in (25.0%:1), while no stain intensities were observed in (87.1%:27) In nodules lesions tissues, the percentages of strong signal intensities of hrHPV18(E7)/PRb-IHC weren’t observed in (0.0%:0), while no staining signal of hrHPV 18(E7)/PRb-IHC was observed in(93.8%:30). Lastly in apparently healthy control, the strong signal intensities of hrHPV18 (E7)/PRb-IHC not detected (0.0%:0). The negative staining signal intensities were deleted in (94.6%:35). Statistically, there is highly significant differences with laryngeal malignant lesions tissues group (p<0.05) as in Table 5 and Figure 2C.
| Studied groups | Rb intensity | X2 (P-value) |
||||
|---|---|---|---|---|---|---|
| NO stain | Strong | |||||
| A.H. Control |
HPV18 E7 intensity |
NO stain | N | 35 | 3 | P=0.679 Non Sign. (P>0.05) |
| % | 94.6% | 100.0% | ||||
| Strong | N | 2 | 0 | |||
| % | 5.4% | 0.0% | ||||
| Total | N | 37 | 3 | |||
| % | 100.0% | 100.0% | ||||
| Nodules Benign laryngeal lesion tissues |
HPV18 E7 intensity |
NO stain | N | 30 | 5 | P=0.565 Non Sign. (P>0.05) |
| % | 93.8% | 100.0% | ||||
| Strong | N | 2 | 0 | |||
| % | 6.2% | 0.0% | ||||
| Total | N | 32 | 5 | |||
| % | 100.0% | 100.0% | ||||
| Polyps Benign laryngeal lesion tissues |
HPV18 E7 intensity |
NO stain | N | 27 | 3 | P=0.515 Non Sign. (P>0.05) |
| % | 87.1% | 75.0% | ||||
| Strong | N | 4 | 1 | |||
| % | 12.9% | 25.0% | ||||
| Total | N | 31 | 4 | |||
| % | 100.0% | 100.0% | ||||
| Malignant laryngeal lesion tissues |
HPV18 E7 intensity |
NO stain | N | 26 | 2 | P=0.017 Sign. (P<0.05) |
| % | 70.3% | 25.0% | ||||
| Strong | N | 11 | 6 | |||
| % | 29.7% | 75.0% | ||||
| Total | N | 37 | 8 | |||
| % | 100.0% | 100.0% | ||||
HrHPV18 (E7) /PRB-IHC score
Also in present results of positive hrHPV18 (E7)/PRB over expression- IHC signal staining with moderate (++) scores in malignant laryngeal lesions were detected in (62.5%:5) and low score(+)(12.5:1) respectively. While the negative signal score was detected in (70.3%:26). In laryngeal polyps lesion tissues, the positive signal score of hrHPV18 (E7)/PRB over expression-IHC with moderate (++) score signal was expressed in (25.0%:1 ).The percentage of negative signal score was (87.1%:27).In the nodules laryngeal lesions tissues, the percentage of hrHPV18(E7)/PRb-IHC with moderate(++) and low(+) signal scores were (0.0%:0). Negative results for these markers were founded in(93.8%:30). In apparently healthy control, there weren’t documented any signal scores of hrHPV18(7)/PRb-IHC in score(++) or score(+) while negative results for these markers constituted (94.6%:35) as in Table 6 and Figure 2D.
| Studied groups | Rb scores | X2 (P-value) |
||||
|---|---|---|---|---|---|---|
| Negative | + | |||||
| A.H. Control |
HPV18 E7 scores |
Negative | N | 35 | 3 | P=0.918 Non Sign. (P>0.05) |
| % | 94.6% | 100.0% | ||||
| + | N | 1 | 0 | |||
| % | 2.7% | 0.0% | ||||
| ++ | N | 1 | 0 | |||
| % | 2.7% | 0.0% | ||||
| Total | N | 37 | 3 | |||
| % | 100.0% | 100.0% | ||||
| Nodules Benign laryngeal lesion tissues |
HPV18 E7 scores |
Negative | N | 30 | 5 | P=0.848 Non Sign. (P>0.05) |
| % | 93.8% | 100.0% | ||||
| + | N | 1 | 0 | |||
| % | 3.1% | 0.0% | ||||
| ++ | N | 1 | 0 | |||
| % | 3.1% | 0.0% | ||||
| Total | N | 32 | 5 | |||
| % | 100.0% | 100.0% | ||||
| Polyps Benign laryngeal lesion tissues |
HPV18 E7 scores |
Negative | N | 27 | 3 | P=0.016 Sign. (P<0.05) |
| % | 87.1% | 75.0% | ||||
| + | N | 4 | 0 | |||
| % | 12.9% | 0.0% | ||||
| ++ | N | 0 | 1 | |||
| % | 0.0% | 25.0% | ||||
| Total | N | 31 | 4 | |||
| % | 100.0% | 100.0% | ||||
| Malignant laryngeal lesion tissues |
HPV18 E7 scores |
Negative | N | 26 | 2 | P=0.00 Highly Sign. (P<0.01) |
| % | 70.3% | 25.0% | ||||
| + | N | 9 | 1 | |||
| % | 24.3% | 12.5% | ||||
| ++ | N | 2 | 5 | |||
| % | 5.4% | 62.5% | ||||
| Total | N | 37 | 8 | |||
| % | 100.0% | 100.0% | ||||
Discussion
Among Head and Neck Squamous Cell Carcinomas (HNSCC), the larynx is one of important sites most taken part in development of cancer and benignneoplasia. Larynx cancer account for>3% of all cancers and considered among the sixth most common cancer worldwide and LSCC is the important [19]. Traditionally, laryngeal lesions related with tobacco smoking and alcohol drinking, especially when in binding with each other in consuming [20]. Squamous cell carcinoma of larynx progresses in a stepby- step manner. Many molecular alterations have been described in various phases of dysplasia [21]. HPV infection particularly high oncogenic risk(Hr- HPV) has been etiologically binding with subsets of laryngeal carcinoma and benign lesions well recognized in recent decades and the frequency of HPV infection among them varies between (0%-79%) [22].
Distribution of age and gender in patients with laryngeal malignant and benign lesions tissues
In current study, as shown in Figure 1, the patients' ages from 8-83 years and the mean age of those with laryngeal malignant lesions tissues was (56.91 ± 17.12) years with highly significance differences from that of laryngeal benign lesions (polyps and nodules) that recorded mean age with forties. While the distribution of age and gender mean in males with laryngeal lesions tissues was higher 110 (43.43%) than in female counter parts 47(39.68%).
The findings of age correspond to those of many other studies, such as those of Akduman who found the affected age by laryngeal carcinoma was 34-84 years and the mean age (57.6) years [23]. Also the present study agreed with Romas Innocentini who found increased laryngeal cancer with age when study attributable factors and the influence on survival rates of Brazilian laryngeal cancer patients, the mean age was (63.29 ± 9.7) year [24]. Contrary, Nachalon disagree with the above mentioned researches claiming that found the mean age 35 ± 5 years in patients with laryngeal squamous cell carcinoma [25].
In benign tumors, the present results agreed with Sadek study who founded the age mean of all studied patients were 41.7 years with a range from 10 to 75 years [26]. Hegde found that laryngeal lesions were common in patients with a mean age of 38 and with maximum affected stratum between 31-40 years [27]. Present study also don’t agree with Upadhgay who demonstrate that the range age of patients with benign vocal cord lesions whose suffering from hoarseness of voice was 31-40 years [28], while Rimoli found the mean age higher than in present results in patients with mean age 67.6 years undergo laryngeal microsurgeries [29]. These studies supported the progression of laryngeal cancer with age. So the similarities and differences of age distribution in patients with difference types of laryngeal lesions could be explained by the prolonged exposure to tobacco smoking, alcohol consumption as well as environmental carcinogens such as chemical materials, radiation and viruses infections, which were regarded as important promoting factors in the laryngeal carcinogenesis [30].
Detection of hrHPV16+18(E6)-IHC/PRb-IHC, hrHPV16 (E7)-IHC/PRb-IHC and hrHPV18(E7)-IHC/PRb-IHC
The study hypothesis use multiple measurements longitudinally to investigate the co-localization of high oncogenic risk HPV16+HPV18 (E6) and (E7) expressions and the expression of one of important tumor suppressor genes product p Rb (Retinoblastoma protein) whose play a fundamental role in regulation of host cell cycle to find an expected role of hrHPV infection in pathogenesis and early steps of laryngeal lesions carcinogenesis [17]. From the results of the present study as shown in Tables 2,4 and 6 it has revealed that pRb over expression and HPV 16+18( E6) and (E7) were significantly higher in laryngeal malignant tumors lesions than in either laryngeal benign lesions( polyps and nodules ) or apparently normal laryngeal tissues through high positive percentages with moderate and low scores in malignant laryngeal lesions for hrHPV16+18(E6)++/ Rb+(50%:4)and(25%:1), hrHPV16(E7)++/Rb+(50%:4),(25%:1) and hrHPV18(E7)++/Rb+(62.5%:5) respectively with strong intensities for all as shown in Tables 1,3 and 5 while low percentage or absence in scores and intensities for these markers in benign laryngeal lesions (polyps and nodules ) as well as laryngeal tissues of apparently healthy control reflecting prevalence of HPV16 and HPV18 with high potential of viral integrated into genome of laryngeal tissue cells with opportunities to increase viral load because of active episomal and integrated viral replication ,also could reflect reactivation of past infection increasing the possibility of hyperplasia and participation in initiated pre-neo plastic mutations and aiding tumor pathogenesis expedite their progression to cancer [31]. Also may indicate that the host's immune system is not efficient enough to clear HPV types, while low percentage of hrHPV16+18(E6)/(E7)/PRb expression may revealed persist infection with episomal replication in benign lesions or may these laryngeal lesions infected with other low risk genotypes of HPV, therefore, these lesions have low level damage in host cell DNA or the damage may be not impair their DNA to induce transcription and translation and regulation of pRb gene [32].When we browsing the scientific literature ,didn’t find a similar study use several parameters to determine the role of hrHPV infection in pathogenesis and tumor genesis and up to present knowledge , this is the first study in Iraq which designed to explain the relationship between hrHPV16, HPV18(E6 and E7) and Rb expression in pathogenesis and carcinogenesis of laryngeal malignant and benign lesions [33].
Conclusion
Over 200 of different types of Human Papilloma Virus (HPV) have been described. High oncogenic HPV16 and HPV18 also have been related to subsets of head and neck squamous cell carcinoma (HNSCC) and laryngeal lesions. It's been reported that elevated levels ofE6 and E7 expressions of hrHPV genotypes as well as tumor suppressor genes expression might have a role in pathogenesis and tumor genesis. This study designed as a retrospective one. The role of human papilloma virus in laryngeal lesions was tested markers in histopathalogical specimens to risk of malignancy is observing significantly in higher (or lower) tendency for laryngeal lesions tissues from malignant cancer to benign lesions tissues (nodules and polyp) to express a positive markers in comparison to apparently healthy laryngeal tissues.
References
- Burd, Eileen M. “Human Papilloma Virus and Cervical Cancer.” Clin Micro biol Rev16 (2003): 1-17.
- Gheit, Tarik.“Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology.” Front Oncol 9 (2019): 355.
- Boda, Daniel, Anca Oana Docea, Daniela Calina, and Mihaela Adriana Ilie, et al.“Human Papilloma Virus: Apprehending the Link with Carcinogenesis and Unveiling New Research Avenues.” Int J Oncol 52 (2018): 637-655.
- Salman, Nadia Aziz, Giles Davies, Farida Majidy, and Fatima Shakir, et al.“Association of High Risk Human Papillomavirus and Breast Cancer: A UK Based Study.” Sci Rep 7 (2017): 1-8.
- Aggarwal, Nikita, Joni Yadav, Kulbhushan Thakur, and Rakhi Bibban, et al.“Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns.”Front Cell Infect Microbiol 10 (2020): 746.
- Dogantemur, Selman, Suleyman Ozdemir, Aysun Uguz, and Ozgur Surmelioglu, et al.“Assessment of HPV 16, HPV 18, p16 Expression in Advanced Stage Laryngeal Cancer Patients and Prognostic Significance.” Braz J Otorhinolaryngol 86 (2020): 351-357.
- Torrente, Avendaño Mariela, Juan P Rodrigo, Missak Haigentz, and Frederik G Dikkers, et al. "Human Papillomavirus Infections in Laryngeal Cancer.” Head Neck 4 (2011): 581-586.
- Mustafa, Mohammed Ahmed, Marwan Q AL-Samarraie, and Marwa T Ahmed.“Molecular Techniques of Viral Diagnosis.”Sci Arch 1 (2020): 89-92.
- Graham, Sheila V. "Human Papilloma Virus: Gene Expression, Regulation and Prospects for Novel Diagnostic Methods and Antiviral Therapies.” Future Microbiol 5 (2010): 1493-1506.
- Duensing, Stefan, Lily Y Lee, Anette Duensing, and John Basile, et al.“The Human Papillomavirus Type 16 E6 and E7 Oncoproteins Cooperate to Induce Mitotic Defects and Genomic Instability by Uncoupling Centrosome Duplication from the Cell Division Cycle.” Proc Natl Acad Sci 18 (2000): 10002-10007.
- AL-Samarraie, Marwan Q, Mohammed K Omar, Arshed H Yaseen, and Mahmood I Mahmood. “The Wide Spread of the Gene Haeomolysin (Hly) and the Adhesion Factor (Sfa) in the E. coli Isolated from UTI." J Pharmaceutical Sci Res11 (2019): 1298-1303.
- Songock, William K, Seong-man Kim, and Jason M Bodily.“The Human Papillomavirus E7 Oncoprotein as a Regulator of Transcription.” Virus Res 231 (2017): 56-75.
- Strati, Katerina, and Paul F Lambert.“Role of Rb-Dependent and Rb-Independent Functions of Papillomavirus E7 Oncogene in Head and Neck Cancer.” Cancer Res 67 (2007): 11585-11593.
- Wiest, Tina, Elisabeth Schwarz, Christel Enders, and Christa Flechtenmacher, et al.“Involvement of Intact HPV16 E6/E7 Gene Expression in Head and Neck Cancers with Unaltered p53 Status and Perturbed pRb Cell Cycle Control.”Oncogene 21 (2002): 1510-1517.
- Jiang, Hua, and Peng-Fang Lin.“Human Papillomavirus Infection a Favourable Prognostic Factor in Laryngeal Squamous Cell Carcinoma is Associated with the Expression of Proliferating Cell Nuclear Antigen.”Pak J Med Sci 29 (2013): 1173.
- Xu, Yanan, Suru Liu, Hongliang Yi, and Jiadong Wang, et al.“Human Papillomavirus Infection in 674 Chinese Patients with Laryngeal Squamous Cell Carcinoma.” PLoS One 9 (2014): e115914.
- Yang, Dongli, Yong Shi, Yemei Tang, and Hongyu Yin, et al.“Effect of HPV Infection on the Occurrence and Development of Laryngeal Cancer: A Review.” J Cancer 10 (2019): 4455.
- Li, Xiangwei, Lei Gao, Huijun Li, and Jing Gao, et al.“Human Papillomavirus Infection and Laryngeal Cancer Risk: A Systematic Review and Meta-Analysis.”J Infect Dis 207 (2013): 479-488.
- Hosseini, Seyed Zinab, Manoochehr Makvandi, Alireza Samarbafzade, and Ali Timori, et al.“Frequency of Human Papilloma Virus (HPV) 16 and 18 Detection in Paraffin-Embedded Laryngeal Carcinoma Tissue.” Asia Pac J Cancer Prev18 (2017): 889.
- Talamini, R, C Bosetti, C La Vecchia, and L Dal Maso, et al.“Combined Effect of Tobacco and Alcohol on Laryngeal Cancer Risk: A Case–Control Study.” Cancer Causes Control 13 (2002): 957-964.
- AL-Samarraie, Marwan Q, Arshed H Yaseen, and Baraa M Ibrahim. "Molecular Study of Polymorphism for Gene tnf-Alpha Using Arms-pcr Technique for Patients with Rheumatoid Arthritis.” Biochem Cell Arch 19 (2019): 4285-4290.
- Nelke, Kamil H, Lidia Lysenko, Jarosław Leszczyszyn, and Hanna Gerber. “Human Papillomavirus and its Influence on Head and Neck Cancer Predisposition.” Postepy Hig Med Dosw 67 (2013): 610-6.
- Akduman, Davut, Murat Karaman, Celil Uslu, and Omer Bilac, et al.“Larynx Cancer Treatment Results: Survive and Quality of Life Assessment.” Kulak Burun Bogaz Ihtis Derg 20 (2010): 25-32.
- Innocentini, Lara Maria Alencar Ramos, Alisson Henrique Teixeira, Luciana Assirati Casemiro, and Matheus Carrijo Andrade, et al.“Laryngeal Cancer Attributable Factors and the Influence on Survival Rates: A Single Brazilian Institution Experience.” Int Arch Otorhinolaryngol 23 (2019): 299-304.
- Nachalon, Yuval, Ohad Cohen, Uri Alkan, and Jacob Shvero, et al. “Characteristics and Outcome of Laryngeal Squamous Cell Carcinoma in Young Adults.” Oncol Lett 13 (2017): 1393-1397.
- Sadek, Ahmed A, Mohamed G Essawy, Ahmed A Abdel-Aziz, and Mostafa M Talaat. “Ultrasonography and Laryngo scopic Findings are Similar in Detecting Laryngeal Lesions.” Egypt J Ear Nose Throat Allied Sci 20 (2019): 137-143.
- Hegde, Mahesh Chandra, M Panduranga Kamath, Kiran Bhojwani, and Ranjith Peter, et al.“Benign Lesions of Larynx—a Clinical Study.” Indian J Otolaryngol Head Neck Surg 57 (2005): 35.
- Upadhyay, Aparaajita, Asiya Kamber Zaidi, and RK Mundra.“A Comprehensive Analysis of Benign Vocal Fold Lesions Causing Hoarseness of Voice and our Experience with Cold Knife Endolaryngeal Surgery in a Tertiary Healthcare Centre.” Indian J Otolaryngol Head Neck Surg 71 (2019): 515-521.
- Rimoli, Caroline Fernandes, Evaldo Dacheux Macedo, Maria Theresa Costa Ramos Oliveira Patrial, and Cynthia Fontoura Klas, et al.“Profile of Laryngeal Microsurgeries in Patients Over 60 Years Old.” Int Arch Otorhinolaryngol 24 (2020): e53-e61.
- Wogan, Gerald N, Stephen S Hecht, James S Felton, and Allan H Conney, et al.“Environmental and Chemical Carcinogenesis.”Semin Cancer Bio 14 (2004): 473-486.
- Liu, B, Z Lu, P Wang, and Z Basang, et al. “Prevalence of High-Risk Human Papillomavirus Types (HPV-16, HPV-18) and Their Physical Status in Primary Laryngeal Squamous Cell Carcinoma.” Neoplasma 57 (2010): 594-600.
- Pal, Asmita, and Rita Kundu.“Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy.”Front Microbiol 10 (2020): 3116.
- Muhsin, Mamdooh A. Al-Nasrawi, and Marwan Q. AL-Samarraie. “The Molecular Sequence of Giardia Lamblia by Using (tpiA) and (tpiB).” Int J Drug Delivery Technol 9 (2019): 374-377.
Citation: Tamara Amer Taha. "Molecular and Virological Study of Nuclear Co-localization of High Oncogenic Risk HPV16 and HPV18 (E6)/ (E7) Genes Products Expressions and Over Expression of PRb Protein in Tissue from Laryngeal Lesions." Clin Schizophr Relat Psychoses 15S(2021). Doi: 10.3371/CSRP.TT.082521.
Copyright: © 2021 Taha TA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.