Research Article - Clinical Schizophrenia & Related Psychoses ( 2022) Volume 0, Issue 0
Mirror Therapy Feedback for Selective Motor Control of the Upper Extremities in Spastic Hemiplegic Cerebral Palsy: A Randomized Controlled Trial
Amira. H. Mohammed1*, Amel. E. Abdel Karim2, Mohamed. H. Abouelenein3 and Samah. M. Sheha22Department of Physical Therapy for Pediatrics, Misr University for Science and Technology, Giza, Egypt
3Department of Physical Therapy for Basic Science, Misr University for Science and Technology, Giza, Egypt
Amira. H. Mohammed, Department of Physical Therapy for Pediatrics, Faculty of Physical Therapy, Delta University for Science and Technology, Gamasa, Egypt, Email: amira_hussin77@yahoo.com
Received: 07-Feb-2022, Manuscript No. CSRP-22-53602; Editor assigned: 09-Feb-2022, Pre QC No. CSRP-22-53602 (PQ); Reviewed: 23-Feb-2022, QC No. CSRP-22-53602; Revised: 28-Feb-2022, Manuscript No. CSRP-22-53602 (R); Published: 07-Mar-2022, DOI: 10.3371/CSRP.MAAK.030722
Abstract
Objective: To detect the outcome of mirror therapy on Selective Motor Control (SMC) of the upper extremities in spastic hemiplegic cerebral palsy.
Design: Randomized Controlled Trial
Setting: Different Pediatrics Rehabilitation Centers and outpatient Clinic of Misr University for science and technology.
Subjects: Forty-five children with unilateral paralysis from both genders participated in this study. They randomised equally into study or control group.
Intervention: The study group received physical therapy program combined with mirror visual feedback from non-affected upper limb. Control group received physical therapy program only. Both groups received the intervention for three successful months.
Measures: All children were assessed using Selective control of upper extremity scale (SCUES). All outcomes were measured at the initial randomization and then after three months of intervention.
Results: The pre-treatment mean value and SD for the total score of the SCUES in the control and study groups were 5.73 ± 1.03 and 5.46 ± 0.92 respectively. The post-treatment mean value and SD for the same variable in the control and study groups were 9.67 ± 1.29 and 11.60 ± 1.06 respectively. So, The Selective Control of Upper Extremity Scale (SCUES) scores revealed statistically significant differences both groups with favour to the study group.
Conclusion: Mirror therapy has a significant effect on SMC of the affected arm in spastic hemiplegic cerebral palsy.
Keywords
Mirror therapy • Selective motor control • Hemiplegia • Children
Introduction
Cerebral Palsy (CP) is a group of long-lasting motor problems of posture and movement caused by brain injury that occurs before, during, or after birth. The injury affects the motor system and results in activity limitations. Hemiplegic CP is the most common type of CP. Children suffering from spastic hemiplegic CP have unilateral impairment in the upper and lower limbs on the same side of the body. This impairment is due to pyramidal tract lesion [1].
These children have neurodevelopmental problems in form of spasticity, poor co-ordination, impaired Selective Motor Control (SMC) and poor balance [2].Inability to initiate the muscles activity in certain pattern in response to the voluntary movement's demands is defined as impaired SMC [3]. A concurrent, compulsory extensor or flexor pattern at two or more joints interfere with the spastic patient’s ability to perform isolated joint movements. So, those subjects produce enforced muscle co-contraction of flexor or extensor muscles when performing the daily living activities [4-6].
Neuro-developmental technique, sensory integration therapy, constraint-induced movement therapy, bilateral therapeutic exercises, and mirror therapy are commonly interventions used in the rehabilitation for children with spasticity [4]. Mirror-mediated therapy act to stimulate the motor cortex. Studies describing the application of mirror therapy in children with CP is so far scarce [7].
This study aimed to detect the outcome of mirror therapy on SMC of the upper limbs in spastic hemiplegic CP. Because of its reduced cost, relative simplicity, and high patient adherence, it could be a valuable adjunct for children with hemiparesis as a home-based motor intervention.
Materials and Methods
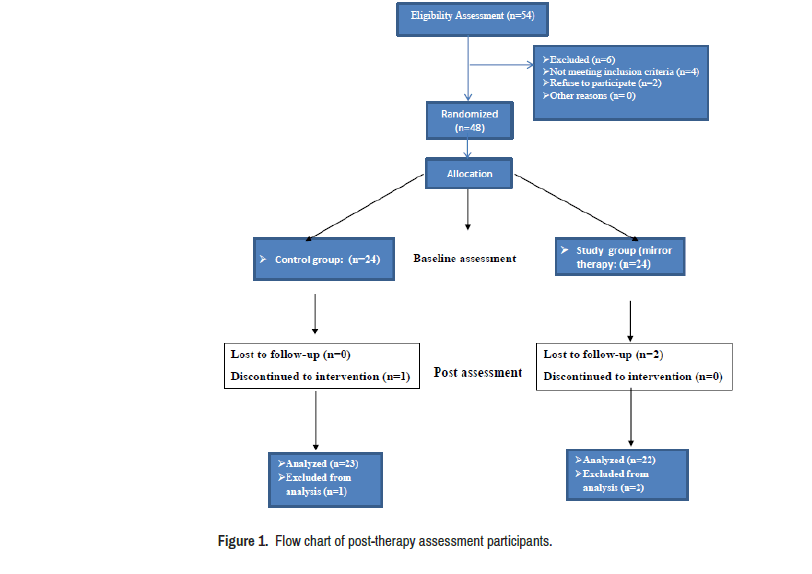
Fifty-four children diagnosed with hemiplegic CP were recruited from different paediatrics rehabilitation centres and the private out-patient clinic of Misr University for Science. They were assessed for eligibility; four participants did not meet the inclusion criteria and two parents refused to participate in this study. Therefore, forty-eight hemiplegic CP children were enrolled in this study they were from both genders, their age ranged from six to nine years, they had mild to moderate spasticity as measured by the Modified Ashworth Scale, and they were able to follow verbal instructions. Children who had any visual or auditory problems, fixed deformities in upper limb that interfere with fine motor functions, history of upper limbs surgery for less than one year, and un-cooperative children were excluded. The Research Ethical Committee, Faculty of Physical Therapy, Cairo University approved this study (P.T.REC/012/003117). The parents of each child signed an informed consent form. Our study was registered on Pan African Clinical Trial Registry (PACTR202105527236004).
The sample size was calculated based on parameters drawn from a prior study which published in 2017. Researchers examine the effectiveness of mirror intervention on the gross motor function in children with spastic CP. The results showed that there was significant improvement in the mirror therapy group versus the control group. The mean and standard deviation value for pre and post intervention in the mirror therapy group was 12.29 ± 3.49 and 19.29 ± 4.11 respectively. While the mean and standard deviation value for pre and post intervention in the control group was 12.86 ± 3.89 and 14.43 ± 3.69 respectively. There were significant differences in the scores obtained from the children with favor to the mirror therapy group. Using these values and our assumption (effect size=0.75 α=0.05 and power=0.8) [8]. We estimated that a sample size of at least 20 participants per group would be required.
The participated children were allocated randomly into two equal groups, using random allocation software to minimize selection bias [9]. Two children dropped out of the post-therapy assessment and one child did not complete the treatment protocol due to travel with parents to another country. Flow chart of participants is shown in Figure 1.
Outcome measures
Upper extremity by SCUES was used to assess SMC for five joint motions of upper limb that includes shoulder abduction/adduction, elbow flexion/extension, forearm supination/pronation, wrist flexion/extension and fingers/thumb grasp/release. Based on the absence or presence of the feature of reduced selective motor control noticed while the subject executed the movement (Limited ROM, excessive joint movement, trunk movement mirror movements) each joint motion was scored on a fourpoints scale to normal SMC, mildly diminished, significantly diminished, and no SMC [8]. The selective motor control for the upper limb joints were assessed at the baseline time and after three months of interventions.
Intervention
Control group (G1): Children in the control group were given 60 minutes of a neurodevelopment-based guideline programme that included stretching activities for the elbow flexors and forearm supinators of the upper limbs, fine motor activities, full range of motion tasks, and finger/ forearm strengthening for the involved side for three sessions/week for three months.
Mirror therapy group (G2): Children received mirror therapy and the same guideline protocol, 60 minutes (30 minutes guideline protocol, 30 minutes mirror therapy three sessions/week for three months). The mirror therapy consisted of 15 minutes basic exercise and 15 minutes functional task.
We used bilateral movement training. The children were instructed to sit in front of the table, put their non-involved limb in front of the mirror and the involved upper limb behind the mirror (A size of 25 × 20 inches) positioned in the mid-sagittal plane at a 70° angle to 80° angle to the trunk. They were instructed to move both limbs in a synchronized manner and to look in the mirror at the reflection of their non- involved upper limb.
For the basic exercise (the first 15 minutes) children were instructed to perform fingers flexion/extension wrist flexion/extension, ulnar/radial deviation forearm supination/pronation elbow flexion/extension shoulder adduction/abduction. For the functional task for (the second 15 minutes) children were asked to perform hand grip exercise, putting blocks into bucket, turning cards, moving a small ball and putty palmar squeezing. To reduce fatigue of the non-affected hand, participants were given a oneminute rest time after each set.
Statistical analysis
Within each group, the paired samples t test was employed to compare variables before and after intervention. The independent t test was utilised to assess pre- and post-test changes in SMC and SCUES score for five upper-limb joint movements between the mirror treatment and control groups.
Results
Descriptive data of age, gender distribution, and the degree of spasticity were represented in Table 1.
| Item | Gender | Degree of spasticity | Age | ||||
| Male | Female | 1 | 1+ | 2 | Mean ± SD | ||
| Group | Control | 9 | 14 | 5 | 11 | 7 | 6.67 ± 0.70 |
| Study | 9 | 13 | 4 | 9 | 9 | 6.80± 0.74 | |
Note: SD-Standard deviation. |
|||||||
Each group showed significant improvements when comparing the preand post-treatment mean values of SMC assessment (Table 2). Comparing the post-treatment mean values of SMC for both groups represent a significant improvement in the total score of SCUES for mirror therapy group as (p<0.05) (Table 3).
| Item | Group | Mean ±SD | MD | t-value | P value | Percentage of improvement | |
| Pre | Post | ||||||
| Shoulder | Control | 1.20 ± 0.41 | 2.53 ± 0.52 | 1.33 | 10.583 | 0.000* | 110.83% |
| Study | 1.33 ± 0.49 | 2.87 ± 0.35 | 1.53 |
11.500 | 0.000* | 117.69% | |
| Elbow | Control | 1.13 ± 0.35 | 1.93 ± 0.60 | 0.80 | 7.483 | 0.000* | 60.15% |
| Study | 1.20 ± 0.41 | 2.27 ± 0.46 | 1.07 |
16.000 | 0.000* | 89.17% | |
| Forearm | Control | 1.20 ± 0.41 | 1.87 ± 0.52 | 0.67 | 5.292 | 0.000* | 55.83% |
| Study | 1.20 ± 0.41 | 2.33 ±0.49 | 1.13 | 12.475 | 0.000* | 94.17% | |
| Wrist | Control | 1.13 ± 0.35 | 1.93 ± 0.26 | 0.80 | 7.483 | 0.000* | 60.15% |
| Study | 0.93 ± 0.35 | 1.87 ± 0.34 |
0.94 |
11.000 | 0.000* | 101.07% | |
| Finger & thumb | Control | 1.07 ± 0.46 | 1.73 ± 0.70 | 0.67 | 5.292 | 0.000* | 62.62% |
| Study | 0.86 ± 0.35 | 1.86 ± 0.35 | 1.00 | 10.247 | 0.000* | 116.3% | |
| Total score | Control | 5.73 ± 1.03 | 9.67 ±1.29 | 3.93 | 21.647 | 0.000* | 68.59% |
| Study | 5.46 ± 0.92 | 11.6 ±1.06 | 6.13 |
25.947 | 0.000* | 112.27% | |
Note: MD-Mean differences; Pre-Pre treatment, Post-Post treatment, *P-value<0.05(significant). |
|||||||
| Item | MD | t-value | P value | |
|---|---|---|---|---|
| Age | 0.133 | 0.487 | 0.63 | |
| Shoulder | Pre | 0.133 | 0.487 | 0.63 |
| Post | 0.333 | 1.950 | 0.061 | |
| Elbow | Pre | 0.067 | 0.475 | 0.638 |
| Post | 0.333 | 1.722 | 0.096 | |
| Forearm | Pre | 0 | 0 | 1.000 |
| Post | 0.467 | 2.544 | 0.017 | |
| Wrist | Pre | 0.200 | 1.775 | 0.087 |
| Post | 0.400 | 2.806 | 0.009 | |
| Finger & thumb | Pre | 0.200 | 1.341 | 0.190 |
| Post | 0.133 | 0.656 | 0.517 | |
| Total score | Pre | 0.267 | 0.748 | 0.461 |
| Post | 1.933 | 4.49 | 0.000* | |
Note: MD-Mean differences; Pre-Pre treatment, Post-Post treatment, *P-value<0.05(significant). |
||||
Discussion
This research was designed to detect the outcome of mirror therapy on SMC of the affected upper extremity in spastic hemiplegic CP. Our results demonstrated a significant improvement in the SMC of the affected upper extremity in both groups with favor to the mirror therapy group [10-12].
These results stand in accordance with Smorenburg, et al. who suggested that the optimistic outcome of mirror visual feedback in arm motor performance for children with unilateral paralysis is not the result from the perception of bilateral symmetrical movement of the upper extremities. They suggested that it was a resultant of the illusion of two symmetrically moving limbs, as well as mirror visual feedback from the unaffected limb [13].
It also confirmed by Farzamfar, et al. who reported that mirror therapy had higher efficiency on gross motor skill than motor training [11]. Also, Park, et al. stated that mirror therapy had an essential role in promotingupper extremity function anddaily living activities in stroke patients [14]. Improvements reported in the study group using Mirror feedback may be due to the increased activation of the neural cells. This includes activation for the Anterior Cingulate Cortex (ACC) on the unaffected hemisphere and the Dorsolateral Prefrontal Cortex (DLPFC) on the affected hemisphere [15].
The ACC plays an important role in motor control [16], the prefrontal cortex deals with non-routine operations [17] and the DLPFC modulate the lower-level systems [18].The DLPFC was activated in response to the augmented attentional request for the assimilation of the vision and proprioception and the need to perform eye hand coordinated tasks [19]. Also, the improvements in the mirror therapy group may be due to the activation of mirror neuron system [20,21].
Conclusion
Mirror visual feedback from the less impaired upper limb of hemiplegic cerebral palsy has a significant effect on selective motor control of the affected upper limb. Mirror therapy has reduced cost, relative simplicity, and high patient [adherence; it could be a valuable adjunct as a home-based motor intervention for children with hemiparesis. Additional research with a longer time frame and post-treatment programme follow-up are needed to ensure the maintenance of the statistical results and emphasis that no loss or relapse in all parameters.
Acknowledgment
We appreciate the cooperation of all parents and their children in this research. We also thank anonymous referees for their useful suggestions.
Authors’ Contribution
Amira. H. Mohammed: Experimental design, technical implementation, data collection, analysis, manuscript preparation and revision.
Amel. E. Abdel Karim: Manuscript preparation, Data analysis and interpretation.
Mohamed. H. Abouelenein: Data collection, manuscript revision.
Samah. M. Sheha: Data collection and analysis, manuscript preparation and revision.
Conflict of Interest
None declared
Role of Funding Source
No advantages or assets were gotten on the side of this research.
References
- Miller, Freeman. "Physical Therapy of Cerebral Palsy." Berlin: Springer, Germany, (2007).
- Lim, Hyoungwon. "Correlation between the Selective Control Assessment of Lower Extremity and Pediatric Balance Scale Scores in Children with Spastic Cerebral Palsy." J Phys Ther Sci 27 (2015): 3645-9.
[Crossref] [Google scholar] [pubmed]
- Sanger, Terence D., Daofen Chen, Mauricio R. Delgado and Deborah Gaebler-Spira, et al. "Definition and Classification of Negative Motor Signs in Childhood." Pediatrics 118 (2006): 2159-67.
[Crossref] [Google scholar] [pubmed]
- Olree, Kenneth S., Jack R. Engsberg, Sandy A. Ross and T. S. Park. "Changes in Synergistic Movement Patterns after Selective Dorsal Rhizotomy." Dev Med Child Neurol 42 (2000): 297-303.
[Crossref] [Google scholar] [pubmed]
- Thelen, Darryl D., Scott A. Riewald, Deanna S. Asakawa and Terence D. Sanger, et al. "Abnormal Coupling of Knee and Hip Moments During Maximal Exertions in Persons with Cerebral Palsy." Muscle Nerve 27 (2003): 486-93.
[Crossref] [Google scholar] [pubmed]
- Cahillâ?ÂÂRowley, Katelyn and Jessica Rose. "Etiology of Impaired Selective Motor Control: Emerging Evidence and its Implications for Research and Treatment in Cerebral Palsy." Dev Med Child Neurol 56 (2014): 522-8.
[Crossref] [Google scholar] [pubmed]
- Park, Eom-ji, Soon-hyung Baek and Soohee Park. "Systematic Review of the Effects of Mirror Therapy in Children with Cerebral Palsy." J Phys Ther Sci 28 (2016): 3227-31.
[Crossref] [Google scholar] [pubmed]
- Wagner, Lisa V., Jon R. Davids and James W. Hardin. "Selective Control of the Upper Extremity Scale: Validation of a Clinical Assessment Tool for Children with Hemiplegic Cerebral Palsy." Dev Med Child Neurol 58 (2016): 612-7.
[Crossref] [Google scholar] [pubmed]
- Saghaei, Mahmood. "Random Allocation Software for Parallel Group Randomized Trials." BMC Med Res Methodol 4 (2004): 1-6.
[Crossref] [Google scholar] [pubmed]
- Gawad, Hala A. Abdel, A. H. Mohammed and A. E. A. Karim. "Shock Wave Therapy for Spastic Plantar Flexor Muscles in Hemiplegic Cerebral Palsy Children." Egypt J Med Hum Genet 16 (2015): 269-75.
- Farzamfar, Pegah, Ali Heirani and Mostafa Sedighi. "The Effect of Motor Training in Mirror Therapy on Gross Motor Skills of the Affected Hand in Children with Hemiplegia." Iran Rehabil J 15 (2017): 243-8.
- Bruchez, Roselyn, Marine Jequier Gygax, Sylvie Roches and Joel Fluss, et al. "Mirror Therapy in Children with Hemiparesis: A Randomized Observerâ?Âblinded Trial." Dev Med Child Neurol 58 (2016): 970-8.
[Crossref] [Google scholar] [pubmed]
- Smorenburg, Ana RP, Annick Ledebt, Max G. Feltham and Frederik JA Deconinck, et al. "The Positive Effect of Mirror Visual Feedback on Arm Control in Children with spastic Hemiparetic Cerebral Palsy is Dependent on Which Arm is Viewed." Exp Brain Res 213 (2011): 393-402.
[Crossref] [Google scholar] [pubmed]
- Park, Jin-Young, Moonyoung Chang, Kyeong-Mi Kim and Hee-Jung Kim. "The Effect of Mirror Therapy on Upper-extremity Function and Activities of Daily Living in Stroke Patients." J Phys Ther Sci 27 (2015): 1681-3.
[Crossref] [Google scholar] [pubmed]
- Weisstanner, Christian, Stefanie Saxer, Roland Wiest and Alain Kaelin-Lang, et al. "The Neuronal Correlates of Mirror Illusion in Children with Spastic Hemiparesis: A Study with Functional Magnetic Resonance Imaging." Swiss Med Wkly 147 (2017): w14415.
[Crossref] [Google scholar] [pubmed]
- Paus, Tomas. "Primate Anterior Cingulate Cortex: Where Motor Control, Drive and Cognition Interface." Nat Rev Neurosci 2 (2001): 417-24.
[Crossref] [Google scholar] [pubmed]
- Miller, Earl K. and Jonathan D. Cohen. "An Integrative Theory of Prefrontal Cortex Function." Annu Rev Neurosci 24 (2001): 167-202.
[Crossref] [Google scholar] [pubmed]
- Heekeren, Hauke R., Sean Marrett and Leslie G. Ungerleider. "The Neural Systems that Mediate Human Perceptual Decision Making." Nat Rev Neurosci 9 (2008): 467-79.
[Crossref] [Google scholar] [pubmed]
- Fink, Gereon R., John C. Marshall, Peter W. Halligan and Chris D. Frith, et al. "The Neural Consequences of Conflict between Intention and the Senses." Brain 122 (1999): 497-512.
[Crossref] [Google scholar] [pubmed]
- Matthys, Koen, Marion Smits, Jos N. Van der Geest and Aad Van der Lugt, et al. "Mirror-induced Visual Illusion of Hand Movements: A Functional Magnetic Resonance Imaging Study." Arch Phys Med Rehabil 90 (2009): 675-81.
[Crossref] [Google scholar] [pubmed]
- Deconinck, Frederik JA, Ana RP Smorenburg, Alex Benham and Annick Ledebt, et al. "Reflections on Mirror Therapy: A Systematic Review of the Effect of Mirror Visual Feedback on the Brain." Neurorehabil Neural Repair 29 (2015): 349-61.
[Crossref] [Google scholar] [pubmed]
Citation: Mohammed, Amira H., Amel. E. Abdel Karim, Mohamed. H. Abouelenein and Samah. M. Sheha. “Mirror Therapy Feedback for Selective Motor Control of the Upper Extremities in Spastic Hemiplegic Cerebral Palsy: A Randomized Controlled Trial.” Clin Schizophr Relat Psychoses 16S (2022). Doi:10.3371/CSRP.MAAK.030722.
Copyright: © 2022 Mohammed AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.