Research Article - Clinical Schizophrenia & Related Psychoses ( 2023) Volume 17, Issue 3
Meningeal Lymphatic Vessels and Glymphatic System in Neurocysticercosis. A Systematic Review and Novel Hypotheses.
Humberto Foyaca Sibat* and Lourdes de Fatima Ibanez ValdesHumberto Foyaca Sibat, Department of Neurology, Walter Sisulu University, Mthatha, South Africa, Email: humbertofoyacasibat1@gmail.com
Received: 28-Feb-2023, Manuscript No. CSRP-23-87304; Editor assigned: 03-Mar-2023, Pre QC No. CSRP-23-87304 (PQ); Reviewed: 20-Mar-2023, QC No. CSRP-23-87304; Revised: 27-Mar-2023, Manuscript No. CSRP-23-87304 (R); Published: 03-Apr-2023, DOI: 10.3371/CSRP.DLHF.040323
Abstract
Background: Neurocysticercosis is an eradicable and preventable zoonotic parasitic neurological disease caused by the infection of the larva form of pig tapeworm Taenia solium, causing headaches and epileptic seizures most seen in developing countries worldwide. When the cysticercus is dying in the Central Nervous System (CNS), many pro-inflammatory elements are realized to participate in the immune response. The primary aid of this article is to look at how waste products from this process are removed from the CNS to the periphery through the Meningeal Lymphatic Vessel (MLV) and the Glymphatic System (GS) supported by aquaporin 4 (AQP4) and Corpora Amylacea (CA). To answer our research question, we conducted a systematic review of the medical literature (never done before) and delivered some hypotheses for each situation.
Method: We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like "NCC and lymphatic CNS", "meningeal lymphatic vessels", "glymphatic system",; OR "NCC/MLS/GS"; OR "waste removal in NCC"; OR "MLS/GS/ Epilepsy;" OR "MLS/GS/neuroparasitology"; OR "Zoonosis and brain LS."
Results: From the total publication, 34 studies were peer-reviewed, and only one discusses issues related to epilepsy and MLV/GS. NCC/MLV/GS studies were reported within the selected review period.
Comments and concluding remarks: We propose a mechanism for clearance of waste metabolic products from cysticercus' destruction, immune response, apoptosis of the neurons, and supporting cells at the CNS in the different stages of NCC supported by MLV, GS, AQP4 and CA. As far as we know, this is the first review made on this topic reported to the medical literature.
Keywords
Meningeal lymphatic vessels • Glymphatic system • Neurocysticercosis • Taenia solium • Epilepsy • Epileptic seizures • Aquaporin 4 • Corpora amylacea
Introduction
Cysticercosis is a preventable zoonosis and eradicable parasitic disease secondary to a cestode infection by the larva form of tapeworm Taenia Solium (Ts), most seen in people living in developing countries.
When the cysticercus is in the cerebral parenchymal, intraventricular system, Subarachnoid Space (SAS), cerebellum, brainstem, optic nerve, or spinal cord, then it has named Neurocysticercosis (NCC), and the often-clinical manifestations are headache and epileptic seizures/epilepsy among other less frequent symptoms and signs. Epilepsy is present in 70-90% of infected cases, and almost all patients respond very well to praziquantel or albendazole as antiparasitic medication and first-line anti-epileptic drugs.
We have investigated many aspects of NCC over the past twenty years, including those related to other associated infectious diseases, and most of our results have been published. Therefore, other interested people can access this information easily [1]. In this study, our central aid is to review the relationship between the NCC and the meningeal lymphatic vessels, the glymphatic system, and aquaporin 4 (AQ4) and to propose novel hypotheses on this issue.
For many years, has been considered that the brain is an immune-privileged organ due to the presence of blood-brain-barrier, Cerebrospinal Fluid (CSF)-brain barrier, among others modulating the relocation of immune cells and based on the lack of Central Nervous System (CNS) drainage [2].
Today is well known that lymphangiogenesis is associated with pathological processes like tissue repair, tumour growth and inflammation, followed by a paradigm shift supported by the discovery of Meningeal Lymphatic Vessels (MLVs) in the CNS. MLVs and those lymphatic vessels located along the dural sinuses primarily absorb CSF from the adjacent SAS and brain Interstitial Fluid (ISF) through the glymphatic system, composed of AQ4 water channels expressed on perivascular astrocytic end-feet membranes.
It is well known that the Cerebrospinal Fluid (CSF) and the Interstitial Fluid (ISF) are the main components of the fluids in the Central Nervous System (CNS). For many years, the medical community believed the CSF (ultrafiltrate of blood plasma) was only secreted in the ventricular system by the choroid plexus. We also believed that after travelling through the ventricular system and the SAS, the CSF is reabsorbed by the subarachnoid villi and drained into the superior sagittal sinus and from here to the general venous circulation. Today is well known that other places produce CSF, like ependymal cells of the Ventricular System (VS) and the Subarachnoid Space (SAS) (20%), and the mechanism of absorption occurs at different levels, including the nasal lymphatic vessels and along some extensions of the brain like olfactory, optic nerves and spinal nerves routes where the Meningeal Lymphatic Vessels (MLV) are present. However, the more critical function of the CSF is to protect the CNS (shock absorption and buoyancy), to keep the homeostasis and accumulation of waste products.
On top of that, the CSF may re-enter the cortex from large arteries/ arterioles by dispersion and from the ventricles enter to the periventricular directly by pulsating flow modulated by changes of the diastolic and systolic arterial pressure along the Virchow-Robin space plus pia and the glia limitans (perivascular space) [3].
15%-20% of the total brain parenchymal is composed of the ISF filling the extracellular space. The main composition of the ISF is neurotransmitters which are relevant to keep the isotonicity of the CNS' cellular microenvironment plus proteins, ions, and peptides. It is known that the source of the ISF is the secretion processes at the BBB. As reported in a previous publication, the relationship between the oncotic and hydrostatic pressure (starling's forces) controls the BBB exudates under pathological circumstances when the protein in serum can get the brain. Other authors confirmed that the ISF's movement through the extracellular space is possible by diffusion and convection mechanisms, although the contribution of each one to the process and the estimation of the quantities of CSF and ISF to be mixed/exchanged remain unknown. However, it has been proved that different brain regions exhibit different diffusion patterns (increased in areas close to the ventricles and depth of the cortical layers. At least three different pathways for ISF drainage have been identified: Through the ependymal cells located in the wall of the VS, the Virchow-Robin space (perivascular) at the brain's surface parenchymal, and the basement membrane at the wall of the blood vessel. There are different places for clearance of the brain's waste, according to some authors, for example: draining solutes from the brain parenchymal occurs from the perivascular pathways into the deep Cervical Lymphatic Nodes (dcLNs), whereas CSF's waste from the SAS and the ventricular system drain to the superficial Cervical Lymph Nodes (sCLNs) and dcLNs. Therefore, changes in the mechanisms of CFS-ISF drainage for whatever reason will cause brain waste accumulation causing neuroimmune reactions.
Historically, the first communication related to the Lymph Nodes (LN) was made in hieroglyphs (around 1600 BC) by ancient Egyptians, and The Alexandrian school and the hippocratic school delivered the functions of the lymphatic system and a better description of the LN. Lymph, named after the Roman goddess "Lympha", means fresh water, was discovered by the ancient Greeks. Lymphatic glands, which are today called lymph nodes, were mentioned in a collection of writings that date to 300 to 500. Other investigators delivered more information about the LS, such as Andreas Vesalius (1543) through his masterpiece 'de Humani corporis fabric. In 1622, Gaspare Aselli reported "several thin and beautiful white cords" in dogs. Years later, Frederik Ruysch (1638–1731) published his finding from anatomical dissection on executed convicts reporting the anatomical features of the valves in the LV and the direction of lymphatic flow [4].
The lymphatic system (including the lymphatic vessels and nodes) was described from 1652 to 1653 by both Swedish (Olaus Rudbeck) and Danish (Thomas Bartholin) physicians. Thirteen years later, Marcello Malpighi in Italy reported the lymphatic function in the spleen, and Paolo Mascagni described with illustration all lymphatic networks around 1784. The first communication regarding the presence of lymphatic vessels in the brain was published in 1787 by Mascagni, a lecturer of anatomy in Siena, Italy. In 1837, J. E. Purkinje (anatomy-physiologist) reported the presence of granular bodies in the brain of elderly peoples and Virchow (1854) made a better description of these bodies named corpora in Latin. In 1869, Schwalbe and collaborators discovered that tracer’s injected intrathecally can be seen later in the LN. Schwalbe was the first to suggest that intrathecally injected tracers appeared in the lymph nodes in 1869. Six years later, Key and Retzius documented the relationship between the nasal mucosa and the CSF space. At the same time (1875), He and colleagues found that the main route of the brain ISF flow is the PVS which Bruce and Dawson highlighted in 1900 after reviewing all available information about spinal lymphatic spaces. At the same time, Bruce and Dawson reviewed the available information on spinal lymphatic spaces. For a long time has been suggested that lymphatic drainage happens through some cranial nerves at the exit of the brain connecting the CFS space and the peripheral LS. Between 1874 and 1990, other authors reported that the lymph space discharged into the PVS while the lymph moved out to the spinal cords periphery. Around 1910, Mott and collaborators announced the presence of lymphatic sheath surrounding the cerebral blood vessels as a continuation of the adventitia delineated by the pia mater covering the neural tissue leading to fusiform cells. In the human meningeal layers (dura matter) investigations, LV was described for the first time in 1987 by Andres and collaborators. It has been confirmed by Aspelund and colleagues and Louveau et al. During this period, the phrases prevascular, perivascular, and paravascular were introduced to named brain lymphatics. In 1992, Cserr and collaborators established that "a new view of nervous system immunology incorporates permanent and highly controlled communication between the immune system and the CNS" after describing the drainage of the CSF via outflow pathways through some cranial nerves and spinal nerve roots. Some relevant discoveries reported between 1990 and 2005 improved our comprehension of the cellular immune reaction in the CNS based on the remarkable role played by the Intercellular Adhesion Molecule-1 (ICAM-1) over the migration of T cells within the nervous system. The same investigators postulated the theory that neutralizing antibodies to ICAM-1 and LFA1 impair the capacity of T cells to cross endothelial barriers in the brain [5].
Before mentioning other historical aspects, it is important to clarify some issues on the before-mentioned Lymphocyte Function-Associated Antigen 1 (LFA1), which is part of the integrin superfamily of adhesion molecules present on leukocytes mainly on lymphocytes playing an important role in the process of emigration where the leukocytes abandon the blood flow to penetrate the nervous tissues, mediates firm arrest of leukocytes, and participate in the process of cytotoxic T cell modulating the killing process of granulocytes and monocytes over antibodies. Since 2007, LFA-1 has six known ligands such as ICAM1, ICAM2, ICAM3, ICAM4, ICAM5, and JAM-A. It is well known that LFA-1/ICAM-1 interactions can stimulate signaling pathways influencing the process of T cell differentiation.
Recently, other authors performing MRI studies in patients looking for clearance pathways in vivo after administration of intrathecal contrast material confirmed that the parasagittal dura next to the superior sagittal sinus act as a bridge between CSF in the brain parenchyma and the Meningeal Lymphatic Vessels (MLV). On the other hand, in anatomopathological studies done on two people who died by self-inflicted hanging, some authors found a very high elevated number of T cells along the afferent branches of the trigeminal nerve compared to two people who died due to a drug overdose and car accident, respectively. These results support the postulate of directional movement of the lymphocyte T cells within the nerve blocked after neck local compression (strangulation) [6].
In animal studies, the before-mentioned waste clearance system, referred to as the glymphatic system of the CNS was discovered in 2012. In 2017, the glymphatic system was first described in the human brain, simultaneously with the first documentation of the meningeal lymphatic network. In addition, other reports on radiological lymphography, MRI, lymphoscintigraphy, near-infrared fluorescence lymphography, and dynamic contrast-enhanced MR lymphangiography studies brought novel information on LS [7].
Last year, Mezey and collaborators performed an immunological study of lymphatic elements in the CNS, and they analysed samples of the trigeminal system present at the lateral wall of cavernous sinus-cavum trigeminal where they found CD3-positive T lymphocytes around ganglionic cells plus large nerves bundles all over like between the fascicles, under the epineurium and perineurium and even in the endoneurial space being all positive for lymphatic markers and the adhesion molecules (ICAM1).
These investigators hypothesize that T lymphocytes move in these spaces engaging in surveillance of the brain's environment. They also postulated that this movement is modulated by the synchronized function of adhesion molecule modulated by epithelial cells and its cognate ligand LFA1 created by immune cells leading to a slow circulation of immune elements along the nerves in the PVCs. These cells are pushed by the movement of the ISF/CSF around the blood vessels or by the endoneurial fluids movements through the fibres of cranial nerves and spinal nerves and travelling from the MLV at the base of the skull or directly from the brain to cervical lymph nodes through the cribriform plate and the nasal mucosa [8].
Finally, we arrive at the last period of CNS lymphatic investigations with novel results like the confirmation of functional flowing LS in the brain identified by specific lymphatic endothelial markers and the discovery of the Glymphatic System (GS), which is composed of the perivascular tunnels lined by the feeding process of astrocytes at the level of the BBB. The main components of GS are the paravenous ISF efflux channel, the para-arterial CSF influx channel, and the water channel Aquaporin-4 (AQP4) in astrocytes connecting the influx and efflux channels. It has been documented that CSF moves through the PVSs of brain arteries toward the PVSs around the venous system pushing the waste elements into the venous sinus system and SAS. The exact direction of the waste movement has been described using structural MRI by Ramirez, et al. These authors describe the clinical consequences secondary to the dysfunctional movement of waste products and the role of the cavernous sinus in the absorption of CSF as well. Recently, lymphatic endothelial markers like PDPN and LYVE1 have been investigated through human brain pathological studies, and the results confirmed that waste products move from the brain IS space to the periphery despite the presence of a neurological condition or not. In all cases, CD3-positive T lymphocytes were present in the lymphatic spaces nearest to lymphatic markers. It was preceded by other investigator's results that employed multiplex immunostainings (tyramide signal amplification) and performed PCRs to localize the markers employed and to document the presence of the mRNA to encode them in one of the samples used for immunocytochemistry. Altogether, support the previous postulate on fluid movement in the spaces in pathological and healthy human’s brain. The findings obtained by Mezey, et al. confirmed the before-mentioned composition of the perivascular space by endothelial cells with the same expression of the peripheral lymphatic endothelial cells Markers (LYVE1, podoplanin, VEGF3, and Prox1, a transcription factor). Podoplanin (PDPN) and LYVE1 are glycoproteins; the first belongs to the mucin-type protein family, and the last is a type I belonging to the integral membrane. They are the primary markers (LYVE1/PDPN) positive cells found all over the brain and both can stain similar cells, although the antigens have different intracellular distributions. VEGFR3 were positive on the same cell while this antibody stained many others, and these cells were positive for lymphatic markers surrounding all vascular cross-sections. In many regions of the human brain have been found perineural and endoneurial staining in cross/longitudinal sections of nerve bundles surrounding satellite cells of the trigeminal ganglion, in the blood vessel in the SAS, dura mater, pia mater, and in the adventitia of the large blood vessels [9].
Other novel historical issues in the acquisition of knowledge on CNS LS are based on lymphogenesis. Now it is well known the LS arises from cells budding off the cardinal veins (anterior and posteriors ones) during the embryogenesis, building up the initial lymphatic network and is also known that those capillaries (lymphatic) are very thin-walled vessels composed of a single layer of lymphatic endotheliocytes without covering by Smooth Muscle Cells (SMC), basement membrane or pericytes with blind ends which function is the drainage of fluids. Contrary to the histological aspect of the endothelial cells of the capillary vessels and their continuous connection, the Lymphatic Capillary Vessels (LCV) have specific intercellular and intermittent connections, which are typically characterized by Parallel Linear Sections (PLS) of vascular endothelial protein cadherin. The gap between these PLS allows the passive passage of fluids and macromolecules direct to the lumen of the vessels. Another recent discovery is the presence of the protein angiopoietin 2 as one of the essential components of LCV structure. Since 2012, has been established that LCV is connected to neighbouring tissue by anchor filaments attached to the interstitial collagen fibres (fibrillin and emilin-1). These filaments participate actively in the mechanism for drainage of the excess extracellular fluids and raise the interstitial pressure stretching the connective tissue fibres leading to an increase in the LCV's diameter and fixing endothelial cells and damaged filaments. If these mechanisms fail, it can cause LS dysfunction like lymphatic leakage, lymphatic drainage disorders and lymphedema. Obviously, at this level, lymphatic endotheliocytes and blood play a relevant role in the mechanisms of new vessel formations after being externally stimulated by growth factors or cytokines [10].
The accuracy of serological investigations for the diagnosis of neurocysticercosis was confirmed in our setting a long time ago. Recently, we recommended a novel approach for patients presenting epilepsy and NCC with associated COVID-19; we also hypothesized on the pathogenesis of seizures, NCC and cytokine release syndrome and the role played by gut microbiota over the medulla oblongata/hypothalamus-pituitary-adrenal axis.
We have hypothesized on the crosstalk of NCC/HIV/AIDS/COVID-19 infections without ARV therapy as a cause of multiple medical consequences and death. Following the arrows is possible to understand the self-explanatory proposal [11].
Finally, we hypothesized the interactions of NCC, HIV/AIDS, and Omicron-SARS-CoV-2 on patients without ARV therapy, as can be graphically represented. The role of SARS-CoV-2 in accelerating the mechanism of T-cell exhaustion and its capacity to decrease the production of INFγ, IL-2, and TNFα should not be ignored in future medical research. Of course, more investigation will clarify doubts and establish curative therapy with safe and sustainable accurate prophylaxis. Recently, we reported a case presenting long-COVID-19 with subarachnoid NCC, associated ischemic stroke, and signs of brainstem dysfunction leading to neurogenic respiratory failure. There is enough evidence to support our hypothesis on the pathogenesis of long-COVID-19, and brainstem dysfunction in patients with NCC, the role of the microbiome and recommended treatment.
Nevertheless, well-designed, double-blinded, randomized controlled trials in SARS-CoV-2 infected patients are needed to confirm further or reject our recommendations. Furthermore, although we investigated the immunologic system and NCC, we never reviewed the relationship between NCC and the CNS lymphatic and glymphatic system. Therefore, how do the LCV and the GS contribute to removing CNS waste in patients with NCC? It is our main research question; therefore, our primary objective will be to look for the necessary information to answer that question.
Materials and Methods
We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like "NCC and lymphatic CNS"; "meningeal lymphatic vessels", "glymphatic system"; OR "NCC/MLS/GS"; OR "waste removal in NCC"; OR "MLS/GS/Epilepsy;" OR "MLS/GS/neuroparasitology"; OR "Zoonosis and brain LS." We also searched a website facility from the US National Library of Medicine for unpublished clinical trials, using the same MeSH terms as above, but applying the filters "full publication" AND "summary", published in English, Spanish, or Portuguese. Inclusion and Exclusion Criteria and Screening process [12].
Publications eligible to be included in this study had to meet the following inclusion criteria:
• Human beings involved.
• The full article was written in English, Spanish or Portuguese.
• The central aspect is NCC, LS, MLS, GS, zoonosis, immunology, neuroimmunology neuroparasitology.
Published in the medical journal after being approved by the peerreview process.
The exclusion criteria were:
• Publication did not refer to issues numbered 3.
• Review articles, letters, medical hypotheses, newspaper publications or manuscripts that did not meet the criteria of an original study.
• Medical conference proceedings.
• Clinical trials with less than ten cases per treatment arm.
• Duplicate articles or manuscript written by the same author using the same data.
• Publication without corresponding authors.
All abstracts were screened twice in a blinded fashion. Those found to meet any exclusion criteria were not included in the analysis, and any discrepancy among authors was solved by close scientific discussion.
Literature search strategy
We included case reports, case series, observational cohort studies, systematic reviews and meta-analyses, cross-sectional studies, and clinical trials. During the initial search, we looked for inclusive articles published between January 01, 2000, and July 30, 2022. We searched the following databases: Science Direct, Google Scholar, Medline, Scopus online databases, Scielo, Search of Sciences, BioRxiv, medRxiv and Cochrane library. All studies were retrieved by utilizing MeSH, as before cited. We did not include other aspects beyond the current work scope [13].
Study and cohort selection
We select prospective and retrospective cohort studies, case reports, case series, case-control studies, controlled clinical trials, reviews, and meta-analysis reporting data on listed topics.
Data collection process
The relevant information was extracted from each publication using Microsoft Excel in a structured coding scheme. The data collected included the type of NCC, clinical features, population size, age distribution, the means used to diagnose NCC, MRI studies for MLS or GS, and Immunological investigations where applicable. In any case, when there was uncertainty regarding the interpretation of the data obtained or how it could be used, the authors discussed the situation in question until they reached a unanimous consensus [14].
Data synthesis
Our investigation used aggregate data where possible, following the PRISMA guidelines.
Quality assessment of included studies
All studies were initially screened for bias using the Jadad scoring system. Trials with a Jadad score<4 were removed; while investigations with a Jadad score ≥ 4 were selected for further assessment [15].
Results
Study selection
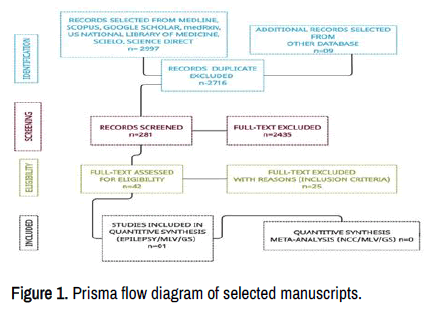
This study aims to update the scientific information released about these issues. A total of 2 997 manuscripts were retrieved from electronic databases up to July 30, 2022. After removing irrelevancy and duplicates, 281 manuscripts were taken for full-text screening, and, finally, 42 publications delivering outcomes of interest were included for review. Of these included studies, 34 were peer-reviewed and only one included epilepsy and MLV/GS reviews. A PRISMA flow chart for the literature searched is shown below Figure 1. None NCC/MLV/GS issues have been reported up to date [16].
Discussion
When writing this manuscript, we could not find any manuscript related to MLS/GS and NCC published in the medical literature. Apart from the publication made by Noe and Marchi on the link between the CNS lymphatic unit, neuroimmunology, and epilepsy, no other studies have been reported worldwide up to date. The before-cited authors established that the MLVs are interconnected with CSF-ISF drainage pathways. This functional mechanism for controlling homeostasis is named CSF-lymphatic unit, which contributes to the interstitial clearance of waste products from the nervous system modulating neuroimmune interactions and the pathogenesis of immune seizure disorders and secondary epilepsies [17].
The postulate of immune-privileged organ for the brain based on its anatomical barriers is today an old history amended by the presence of functional lymphatic vessels in the Meningeal Layers (MLV) of the brain and spinal cord and their role in drainage of solutes/macromolecule from the brain, and the route of communication between the Immune System (IS) in the CNS and a well-defined GS.
A long time ago, we reported several patients presenting active NCC at the vesicular stage who were free of neurological manifestations for many years; we also described the clinical manifestations seen at the colloid stage of NCC when the larva stage of the taenia solium is dying due to natural causes or antiparasitic treatment, including the radiological features observed on CT scan of the brain.
The presence of pro-inflammatory cytokines, chemokines, and macromolecules derived from the colloid process of NCC provide a pre-lactogenic environment at the cortical grey matter leading to a hypersynchrony discharge at the initial axonal segment expressing clinically as epileptic seizures [18]. Below, we will analyse the implication of MLV in the neuroimmune mechanism responsible for realizing CNS derived antigens and drainage of waste material from cysticercus' dying process and immune cells as a critical player in immune surveillance (Figure 2).
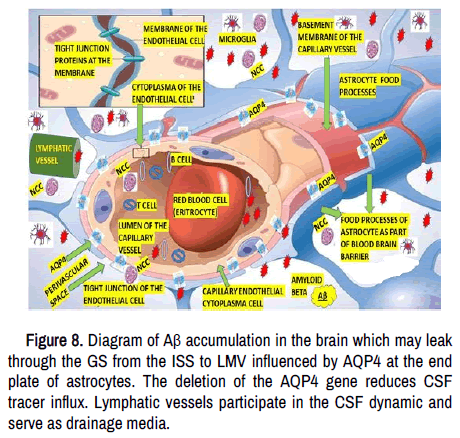
Both fluids interchange across the ependymal tissue in the ventricular system, VR space or basement membrane of the capillary vessel. Amyloid beta (Aβ) and other Waste Metabolic Product (WMP) are drained from the SAS arachnoid villi (CSF), ISF (corpus callosum, anterior commissures and stria terminalis), ISS and VR space surrounding penetrating arterioles via MLV/GS and lymphatic vessels in the nasal cavity, all together supported by Corpus Amylacea (CA) and aquaporin 4 (AQP4) then transported to the cervical lymph nodes where are phagocyted by macrophages. Th2 mediated under Treg regulation, cytotoxic CD8+, Antigen-Presenting Cell (APC), type 2 CD4+ helper is also represented in this Figure.
During normal cell metabolism, Beta-amyloid (Aβ) is produced, a polypeptide of 39-43 amino acids in the blood plasma, CSF, cerebral intercellular fluid, and ISF. The concentration of Aβ in the CNS is regulated by the mechanisms of outflow/inflow of the fluids. Using receptors and transporters like apical-side endothelial RAGE (receptor for advanced glycation end-products), efflux transporters P-glycoprotein/ABCB1 and BCRP/ABCG2, unbound Aβ can pass across the BBB [19].
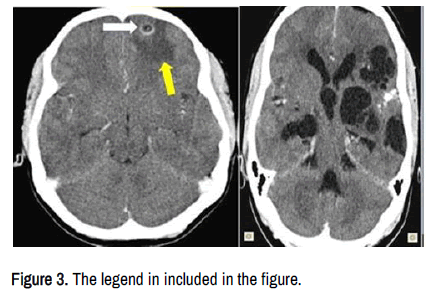
We have hypothesized that MLV drains solutes and cell-associated antigens from the brain parenchymal tissue around to the grey/white junction (most specific location of NCC), interstitial fluid from cysticercus perilesional oedema as shown in Figure 3.
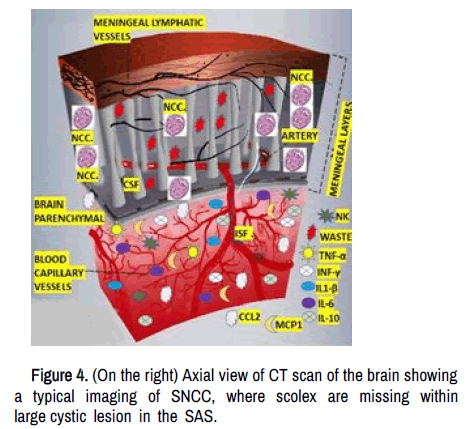
In cases of SNCC (racemose), the metabolic waste material, including the remnant of the scolex, other solutes, dendritic cells, Aβ and macrophages in the CSF, can be drained directly through the MLV, which are in a highly closed relationship with the SAS into the depth and superficial cervical lymph nodes (dCLN/sCLN) being the dCLN the foremost collector of MLV lymph, where B and T's cells integrate this material via specialized antigen-presenting cells (APCs) as is shown in Figure 4.
On the left: Graphic representation of the circulation of the MLV in the duramatter, in closed contact with the CSF in the SAS where the scolex from SNCC degenerated after penetrating the CSF into the cyst increasing their diameter up to 50 mm or more. The waste metabolic material from this process is moved from the SAS via MLV to s/dCLN. The mechanical compression over the brain parenchymal cause neuroinflammation and immune response with accumulation of pro-inflammatory elements (represented in the Figure), neuro apoptosis, death supporting cells among other waste metabolic material which is drained to the CLN via the GS.
In patients with IVNCC, a passage of fluids can be done across the ependymal tissue causing interstitial oedema around the obstructive hydrocephalus, and waste material from the destructive process of the scolex in the lateral, third or fourth ventricle could be drained through the VR perivascular space via GS, cranial nerves II, V, IX, X and XI and even pass through the cribriform plate at the anterior fossa into the lymphatic vessel at the nasal cavity and surrounding lymph nodes as it shows in Figure 5.
To avoid autoimmunity, an effector response is necessary to provide apoptosis from the combined activity of lymphocytes, APCs and CLN and naïve lymphocytes production. Therefore, as other authors have established, the MLV plays a pivotal role in immune-cell activation and cell differentiation, brain fluid movement, drainage of solutes, and participation in the pathogenesis of neurodegenerative disorder secondary to the accumulation of macromolecules and neuro-immune crosstalk [20].
In the brain, the MLV are present in the dura mater facing the SAS, lining the dural sinuses on the calvarium, the middle meningeal arteries at the base of the skull, the pterygopalatine region, and along the spinal nerves and cranial nerves II, V, IX, X, XI. Its principal function is related to interstitial solute clearance, brain homeostasis, immune surveillance, and modulation of neuro-inflammatory response.
The presence of perilesional fluid in NCC is due to local BBB damage causing vasogenic/cytotoxic cerebral oedema. The BBB allow pass through only small molecules like water, lipophilic substances, and some gases by passive diffusion but, under these circumstances, leads to sustained ictal activity, as has been proved by other authors.
Suppose the intraparenchymal cysticercus is dying in that case, there are elevated concentrations of pro-inflammatory elements, with consequent cytokine storm, plenty of antigens, and antibodies plus the end plate astrocytes lesion where are their water channel receptors (to be discussed later) and dysfunctional GS causing:
• Significant fast disequilibrium of ionic concentration (mainly K+) which affect the initiation of the action potential,
• An essential accumulation of serum protein, waste material,
dysfunctional ISF circulation around the perivascular and brain
tissue promoting neuronal hyperexcitability, cell damage,
elevate glutamate concentration, hypometabolism, hypoxia,
perilesional oedema and neuroinflammation contributing to the
production of hyper synchronic discharge of the affected
cortical neurons causing epileptic seizures (Figure 6).
Figure 6. On the right: Axial view of CT scan of the brain show bilateral and multiple active cysticercus in vesicular stage where the density of the intracystic fluid is the same of the CSF in the ventricular system. Yellow arrows show a cyst at the beginning of colloid stage. Although the eccentric scolex is still present, the density of the intracystic fluid increased compared with other cysts, there is an associated swelling of the pericystic tissue in the head of the right caudate nucleus plus a calcified lesion below the cyst.
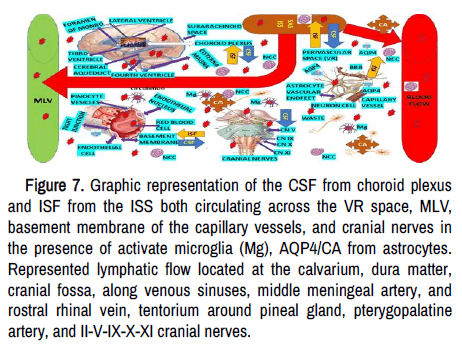
The before-cited astrocyte end-plate axonal damage and the capillary endothelial cell lesion lead to protein accumulation in the interstitial space and water entry into the brain parenchymal with lipophilicity of the Virchow-Robin (VR) perivascular space. Dysfunctional circulation of ISF, which contain ions, proteins, peptides, and neurotransmitters (to keep the isotonicity of the nervous tissue), may cause interstitial accumulation of hyperphosphorylated tubulin-associate protein and pTau (waste products) activating astrocytes and microglia leading to neuroinflammation and epileptic seizures as we proposed recently. As is shown in Figure 7, ISF moves through the extracellular space by diffusion and convection mechanisms. However, mixing and interchange processes between ISF and CFS vary according to the regions of the brain where it happens, like close to the ventricular system and depth cortical layer, and the CSF moves by diffusion. While the ISF moves through ependymal cells in the ventricles, through Virchow-Robin (VR) perivascular space (ISF-CSF exchange) and the basement membrane of the capillary wall (direct flow of ISF to MLV).
Figure 7. Graphic representation of the CSF from choroid plexus and ISF from the ISS both circulating across the VR space, MLV, basement membrane of the capillary vessels, and cranial nerves in the presence of activate microglia (Mg), AQP4/CA from astrocytes. Represented lymphatic flow located at the calvarium, dura matter, cranial fossa, along venous sinuses, middle meningeal artery, and rostral rhinal vein, tentorium around pineal gland, pterygopalatine artery, and II-V-IX-X-XI cranial nerves.
We assume that clearance of waste from the destruction of cysticercus at the brain parenchymal occurs along the VR pathways into the dCLN while the waste material from intraventricular NCC dying process (IVNCC) and destruction of the scolex in subarachnoid NCC (SNCC) drain to sCLN and dCLN.
In our opinion, in patients presenting massive NCC (Figure KLM), the CFS-ISF drainage does not work due to obstruction of MLV, causing unusual pro-inflammatory situations secondary to cell and solute accumulation promoting deadly neuroimmune reactions.
Based on investigations made on animal models by Aspelund et al. and Louveau et al., we know that cleared ovalbumin from the brain moves to the dCLV through the MLV at the base of the brain.
Therefore, considering the same pathway for clearance of interstitial molecules during the colloid stage of NCC, it makes sense. Nonetheless, it has been proved that accumulation of pTau is present in cases with secondary epilepsy, temporal lobe epilepsy, cortical dysplasia, and its participation in the mechanism of neuronal hyperexcitability. Based on those findings, we have hypothesized the pathogenesis of epilepsy secondary to NCC, considering the accumulation of pTau as the main contributing factor to the ictal activity seen in NCC cases when the MLV and GS fail to be draining it to the dCLN.
On top of that, the role of CD8+ T cell activation in the mechanism of adaptive autoimmunity should be highlighted.
We speculate that mechanical obstruction of MLVs by waste products, as suspected in massive brain NCC could result in sending most of all brain-derived antigens toward other lymphatic organs (e.g., spleen, sCLN, or lumbar lymph nodes), affecting the regulation of the neuroimmune response provided by the dcLNs leading to a significant cytotoxic CD8-mediated autoimmune reaction and death, as happened in our case (Figure 8).
Based on the previous report, we propose that NCC be considered autoimmune-mediated CNS inflammation-causing immune epilepsy.
Some authors classified autoimmune encephalitides as:
• T-cell diseases against intracellular antigens (e.g., GAD65);
• Anti-NMDA (N-methyl-d-aspartate) receptor, anti-LGI1, anti-VGKC
complex);
• Encephalitides associated with other autoimmune disorders (e.g.,
lupus cerebritis);
• Encephalitides with pathogenic antibodies against cell surface
proteins. Therefore, immune therapy should be added if patients
do not respond correctly to anti-seizure/anti-epileptic drugs.
Probably, the before-cited CD8+ mediated immune response is due to poor MLV drainage or obstruction of the MLV/GS flow with a consequent loss of neurons and supporting cells. Once more time, we like to highlight that according to the immune capacity of the host, the number, location, and stage of cysticercus in the CNS, and the underlying or comorbidity disease, among other factors, is the amount of releasing pro-inflammatory cytokines, which can upregulate the expression of ICAM-1, VCAM-1, and E-selectin (adhesion molecules) on endothelial cells as have been reported many years back.
Nevertheless, we considered the cysticercus dying process can decrease CNS-lymphatic unit efficiency (activating autoimmune T cells), produce local neuroinflammation, and BBB damage promoting recruitment of CNS B/T lymphocytes under a dynamic process where MLV can be replaced via VEGFR-3 signalling from vascular endothelial growth factors (VEGF-C and VEGF-D), podoplanin, tissue-infiltrating inflammatory cells (e.g., CD11 b+/Gr-1+ macrophages) and tube-like structures among other components. These newly formed MLVs will restore the fluid drainage to dCLN and counteract the inflammatory process caused by dying cysticercus and local cell apoptosis. Other investigators have confirmed that injections of adenoviral VEGF-C vector into the parenchymal tissue and ventricular system induce the growth of MLV, which supports our hypotheses.
Role of TNF in NCC
One of the most relevant neuroimmune elements in the brain is the TNF-α, a cytokine associated with type 1 immunity, made by many types of local cells. It is well known that Tumour Necroting Factor (TNF) signalling has the capacity of restricts neurogenesis and maturation of dendrites and even modulate cognitive behaviour in adults, modulate visual experience, activity-dependent synaptic refinement (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) trafficking, synapsis stability, and restriction of synaptic plasticity. Based on the previous reports, we have considered that TNF plays a relevant role at the colloid stage of the larva stage of Ts in the CNS as a pro-inflammatory component in the epileptogenesis mechanism being the essential neuroimmune element of this process, only overpassed by IL-6 (cytokine storm) in the comorbidity of NCC/COVID-19.
Microglia and NCC
Seems to be that oligodendrocytes are the supporting glial cells less affected directly by NCC based on the lack of areas of demyelination seen in imagenology. However, as mentioned below, CNS myeline damage can be expected secondary to dysfunctional microglia cells. On the other hand, impairment of all types of astrocytes in NCC is remarkably relevant as a component of BBB in the healing process of the nervous tissues during the nodular/fibrotic stage of NCC and the participation of AQP4, which be discussed later. Now, we like to comment on microglia (Mg).
Approximately ten per cent of the brain cells are composed of Mg, which are ramified tissue-resident macrophages (homeostasis) with high participation in the local immunity around cysticercus lesions, mainly at the brain parenchymal. Mg is a phagocytic vesicle that leads to the clearance of apoptotic neurons and other glial cells with enormous sensitivity to immune signals, an extensive lifespan (more than four years), which derive from primitive macrophages and originate in the yolk sac, moving to the fetal head colonizing the brain parenchymal for life without contribution from circulating cells, being programmed by the brain environment (tonic TGF-β signalling) and adopting a CNS-specific stage able to perform highly immune activities during homeostasis, brain development and neurological disorders. The role played by Mg on myelogenesis, oligodendrocyte maintenance, synaptic pruning, remodelling synapses by presynaptic trogocytosis and spine head filopodia induction and inducing neurotoxic reactive astrocytes have been reported. Neuronal cell death is present in different NCC stage modalities where the role of the Mg clearance function is crucial to keep the brain activities within their normal parameters, although this function has not been proven to date. Microglial-secreted pro-inflammatory mediators (IL-α, TNF, and C1 q) induce a reactive astrocyte state, decreased synaptogenesis factors, phagocytic activity, and cytokines produced by astrocytes affect microglia function mailing during homeostasis and inflammatory process as well. The role of microglia activation in NCC- associated COVID-19 was reported recently by one of us. Based on our systematic review of the medical literature, we have more evidence to highlight our previous postulates.
Role of astrocytes/aquaporin-4 (AQP-4) and GS in NCC
How astrocytes activation modifies the CNS local immunological response in patients presenting NCC/COVID-19 was reported in detail before. Now we are to comment on the role of AQP-4 in NCC. The end-feet transmembrane protein water channel facilitates the bidirectional movement of water through the cell membrane. It is considered the primary regulator of water homeostasis of the CNS, and its participation in transmembrane diffusion of some solutes, cell adhesion, cell volume regulation, molecular transportation, and membrane protein expression has been well documented. From the group of AQP, which participate in the regular redistribution of CSF during glucose metabolism in neuron cells? The AQP-4 is the most expressed in the brain, playing a crucial role in the clearance of waste products from disintegrating parasitic process plus surrounding solutes composed of dead neurons and supporting cells, macrophages, plus other molecules and fluids from the ISS and VR space into the capillary vessel via dCLN. Another undesired component to be drained is astrocyte-neuron lactate products from the parasitic local inflammation, which is also transported out of the brain via AQP4-dependent GS clearance through CSF at the SAS, cribriform plate and cranial nerves II, V, IX, X and XI predominantly. Voluntary exercise increases AQP4 expression and clearance of amyloid-β. Based on previously listed evidence, we theorize that the inflammatory response created by the destruction of cysticercus in the CNS can cause depolarization of AQP4, leading to glymphatic flow inhibition, as reported in patients after status epilepticus. The same author reported cerebral oedema attenuation facilitates GS recovery. Based on that evidence, we propose to include anti-cerebral oedema therapy in patients presenting NCC with surrounding perilesional oedema to facilitate the clearance process of remnants of death parasites, apoptotic neuron/supporting cells, deposition of pTau and interstitial fluids. As additional information, we can mention that patients presenting a subtype of dementia syndrome (Idiopathic normal pressure hydrocephalus) loss of AQP4 polarization and GS impairment have been confirmed.
In SNCC (racemose), an associated ischemic stroke due to NCC vasculitis is seven times more often than in the general population. We have hypothesized that cerebral oedema in those cases includes cytotoxic oedema, vasogenic oedema (BBB breakdown), ionic oedema due to impairment of the GS and decreased AQP4 expression and polarization leading to CSF flow into the ischemic area through the VR space. On top of altered AQP4 polarization, a considerable accumulation of immune cells (mainly cytokines) and metabolic waste, including amyloid-β in the VR space during the inflammatory process associated with the destruction of the cysticercus cellulose, can block the GS flow and influx of CSF perpetuating neuroinflammation. In our opinion, the brain's drainage disorder in NCC increases the neuroinflammatory response secondary to the increased accumulation of metabolic waste products and pro-inflammatory elements.
Recently, Kitchen and collaborators reported good results using trifluoperazine to eliminate cerebral oedema by inhibiting calmodulin which drives AQP4 astrocyte cell-surface localization by binding AQP4's carboxyl, and Liu et al. recommended glibenclamide to reduce cerebral oedema due to status epilepticus which inhibit the SUR1-TRMP4 channel complex at the membrane of protoplasmic astrocytes re-establishing the GS regular activity. Up to date, no clinical trial to confirm these recommendations has been performed, but these results bring undoubling novel insight to encourage a look for a new target treatment for NCC AQP4 disorder soon.
The GS supports CSF currents into the brain along with the VR spaces and then into the ISS of the brain through aquaporin4. The dysfunction of the GS may be associated with the influx of CSF, which depends on arterial pulsation and an increased inflow, then more solutes are transferred to the VR space, but they cannot leave the VR space due to the depolarization of aquaporin-4 and dysfunctional GS.
Furthermore, if Aβ accumulates inside the brain parenchymal and vessel walls, it can narrow the VR space with blockage of the GS' cleansing pathway.
In summary, the GS is a fluid-transport system that accesses all over the brain facilitating the exchange of CSF and ISF, clearing waste metabolic products from active CNS. The aquaporin-4 water channels promote fluid exchange between the VR spaces and the neuropil then CSF and ISF are transported back to the VR compartment by meningeal and CLV. Therefore, the MLV drain the CSF and ISF downstream of the GS. To combat neuroinflammation to treat GS dysfunction is mandatory. The homeostatic recovery process and circadian pacemaker process are during sleeping time, and the glymphatic inflow is also related to NREM.
Novel aspect published in the medical literature is related to the modulation of the GS inflow. One of them is the influence of sleep on the CNS clearance system.
The physiological state of decreased arousal has different stages. During the Non-Rapid Eye Movement (NREM) sleep, most CFS drain into ISS, being more efficient in the clearance of solute/metabolite waste compared with the awake stage. Therefore, during wakefulness, the extracellular level of solutes/metabolites waste products increases in the CNS, including the production of Tau oligomers.
Therefore, lack of sleep or sleep/wakefulness cycle disorders represented by an increased level of neuropeptide orexin, deposition of Aβ in the brain, oxidative stress, disruption of the BBB, and circadian rhythm disruption must be considered as a risk factor for several brain pathologies. If the food taken has a high content of salt, refined sugar, fat and animal proteins and is low in vegetables and fruit, then this risk is remarkably higher; at the same time, the diet is capable of restoring the average concentration of cortisol it will provide normal sleep/wakefulness process, better glymphatic clearance and less chance to develop cognitive impairment and dementia syndrome. Obviously, in a patient with NCC, an adequate diet will provide better outcomes and fewer complications, including status epilepticus or stroke, among other problems.
The suprachiasmatic nucleus of the hypothalamus controls the Circadian Rhythm (CR). The function of the CR is to act as a pacemaker in sleep regulation and modulates the GS, CSF production, BBB permeability, and plasma norepinephrine concentration.
A recent discovery confirmed that the diminished glymphatic inflow following sleep deprivation is associated with declined polarization of the AQP4 at VR space, highlighting the previous postulates.
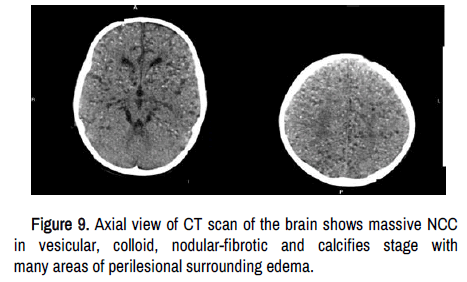
Recently, we reported the role played by the Autonomic Nervous System (ANS) and dysbiosis in patients presenting NCC/COVID-19, including the role of the tenth cranial nerve at the interface of the microbiota–gut–brain axis and as a crucial link between peripheral and central immunity, the repercussion of disbalance between firmicutes/bacteroides on NCC/epilepsy outcome, and how gut microbiome modulates the peripheral immune responses in NCC/ COVID-19/Epilepsy and the immune type II in the gut are responsive to signal sent by the ANS. Now, we like to comment on the sizeable modulatory effect of the ANS over the GS (inflow), as some authors proved refreshing that during normal sleep, the sympathetic tone decreases, whereas the parasympathetic one increase. After activation of γ-aminobutyric acid (GABA) and galanin receptors with active projective inhibition of synapses in the activating reticular formation system, sleep initiation occurs together with the activation of the GS and the role of xylazine and dexmedetomidine blocking the release of norepinephrine from locus coeruleus after binding to the α-2 adrenergic receptors. Neuropeptide neuromedin U from cholinergic parasympathetic neurons promotes type II remodelling responses. It is essential to keep in mind that norepinephrine is the most important mediator of sympathetic 'fight or flight responses and it can suppress ILC2 function and type II immunity which, together with the disturbed process of clearance of solutes/metabolites from cysticercus metabolic decrements during the NREM sleep, increase the risk of developing ischemic stroke associated with NCC vasculitis in IPNCC/SNCC, increase the frequency of recurrent and uncontrolled epileptic seizures and status epilepticus or even death in massive NCC (Figure 9).
Corpora amylacea in NCC
Corpora amylacea (CA) is a granular body composed of 88% of polymerized hexoses (primarily glucose), glycogen synthase for polyglucosan, ubiquin, protein p62, and other cellular components. Astrocytes produce CA, capable of trapping/sequestering/collecting hazardous products from cellular metabolism, plus other waste material from different areas of the CNS. Most CA is expressed in subpial, VR space, and periventricular regions, where they can be released into the CSF/ISF and travel via MLV/GS into the s/dCLN, where macrophages phagocyte them. Considering CA are in periventricular, VR space, and subpial regions of the brain and knowing that the GS drains ISF from the VR space to the CSF, which is close to the ventricles and subarachnoid space, some authors have proposed that CA are expelled from the brain to the CSF. Based on this evidence, we have hypothesized that all solutes/metabolite waste material produced by the disintegration of cysticercus in the CNS, including dead neurons/supporting cells, fragments of cysticercus, immunological material, degenerating mitochondria, membranous elements, and harmful fluids which are incorporated into CA and transported to s/dCLN via MLV where are phagocyted by macrophages.
Conclusion
Unfortunately, NCC is more prevalent in large, disadvantaged countries where research facilities are not good enough to perform extensive neuroimmunology investigations. In our setting, it is not possible because the prevalence of NCC have been decreasing gradually. At the beginning of our studies (20 years ago), NCC/epilepsy was a very often public health problem, and now few patients are confirmed yearly, so the number of cases is scanty. Currently, other African countries with poor resources but receiving scientific support from developed countries will have the capacity to perform the proper research on neuroepidemiology, neuroimmunoparasitology and clinical trials to obtain the necessary results to eradicate completely the diseases before it continue spreading worldwide due to the expected globalization and the current massive emigration.
Consent for Publication
Written informed consent from our patient was obtained for publication, including accompanying diagnostic results. Any interested reader can obtain it by request.
Ethical Approval
The WSU/NMAH institutional ethical committee did not consider this report for additional ethical approval.
Competing Interest
The authors declare that they performed this study without any commercial, financial, or otherwise relationships able to construe a potential conflict of interest.
Funding
Both authors declare that they never received financial support or personal collaboration that could have influenced the results reported in this paper.
Authors' Contributions
Study concept and design: HFS and LFIV.
Data collection from searched literature: LdeFIV and HFS.
Analysis of the obtained data: LdeFIV/HFS.
Drafting of the manuscript: LFIV, HFS.
Revising the manuscript: HFS and LFIV.
Supervised research and manuscript writing process: HFS and LFIV. Both authors have the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the work's integrity, and approve this version to be published.
Declaration of anonymity: Both authors certify that they did not reveal the names, initials, and other identity issues of this patient in this publication, and complete anonymity is guaranteed.
Availability of data and material: The data supporting this study's findings are available on reasonable request from the corresponding author.
Acknowledgement
Special thanks to Dr Sibi Joseph from the Department of Neurology, NMACH. Mthatha, South Africa, for his support.
References
- Foyaca-Sibat, Humberto, and L de F Ibañez-Valdés. “Pseudo Seizures and Epilepsy in Neurocysticercosis.” Electron J Biomed 2 (2003):79-87.
- Foyaca-Sibat, Humberto, and L de F Ibañez Valdés. “Vascular Dementia Type Binswanger’s Disease in Patienys with Active Neurocysticercosis.” Rev Electron Biomed / Electron J Biomed 1 (2003):32-42.
- Foyaca-Sibat, Humberto, and LdeF Ibañez-Valdés. “Insular Neurocysticercosis: Our Finding and Review of the Medical Literature.” Int J of Neurol 5 (2006):2.
- Foyaca-Sibat, Humberto, Linda D Cowan, Hélène Carabin, and Irene Targonska, et al. “Accuracy of Serological Testing for the Diagnosis of Prevalent Neurocysticercosis in Outpatients with Epilepsy, Eastern Cape Province, South Africa.” PLoS Negl Trop Dis 3 (2009):e562.
[Crossref][Google Scholar] [PubMed]
- Foyaca-Sibat, Humberto, L Ibañez-Valdés, and Moré-Rodríguez J. “Parasitic Zoonoses of the Brain: Another Challenger?.” Int J of Neurol 12 (2009):9-14.
- Foyaca-Sibat, Humberto, and L de F Iban~ez-Valde´s. “Treatment of Epilepsy Secondary to Neurocysticercosis.” Nov Treat of Epilep (2011).
[Crossref]
- Foyaca-Sibat, Humberto, and L de F Iban~ez-Valde´s. “Clinical Features of Epilepsy Secondary to Neurocysticercosis at the Insular Lobe.” Nov Treat of Epilep (2011).
- Foyaca-Sibat, Humberto. “Epilepsy Secondary to Parasitic Zoonoses of the Brain.” Nov Aspec Epilep (2011).
- Ringstad, Geir, and Per Kristian Eide. “Cerebrospinal Fluid Tracer Efflux to Parasagittal Dura in Humans.” Nat Commun 11 (2020):354.
[Crossref][Google Scholar] [PubMed]
- Lyck, Ruth, Yvonne Reiss, Nicole Gerwin, and John Greenwood, et al. “T-Cell Interaction with ICAM-1/ICAM-2 Double-Deficient Brain Endothelium In Vitro: The Cytoplasmic Tail of Endothelial ICAM-1 is Necessary for Transendothelial Migration of T Cells.” Blood 102 (2003):3675-3683.
[Crossref][Google Scholar] [PubMed]
- Greenwood, John, Claire L Amos, Claire E Walters, and Pierre-Olivier Couraud, et al. “Intracellular Domain of Brain Endothelial Intercellular Adhesion Molecule-1 is Essential for T Lymphocyte-Mediated Signaling and Migration.” J Immunol 171 (2003):2099-2108.
[Crossref][Google Scholar] [PubMed]
- Marieb, E, and Katja Hoehn. “Human Anatomy and Physiology.” Benjamin Cummings (2009).
- Sobel, R A, Mitchell ME, and Fondren G. “Intercellular Adhesion Molecule-1 (ICAM-1) in Cellular Immune Reactions in the Human Central Nervous System.” Am J Pathol 136 (1990):1309-1316.
[Google Scholar] [PubMed]
- Engelhardt, Britta, and Richard M Ransohoff. “The Ins and Outs of T-Lymphocyte Trafficking to the CNS: Anatomical Sites and Molecular Mechanisms.” Trends Immunol 26 (2005):485-495.
[Crossref][Google Scholar] [PubMed]
- Mezey, Éva, Ildikó Szalayova, Christopher T Hogden, and Alexandra Brady, et al. “An Immunohistochemical Study of Lymphatic Elements in the Human Brain.” Proc Natl Acad Sci U S A 118 (2021):e2002574118.
[Crossref][Google Scholar] [PubMed]
- Goldmann, Jana, Erik Kwidzinski, Christine Brandt, and Jacqueline Mahlo, et al. “T Cells Traffic from Brain to Cervical Lymph Nodes via the Cribroid Plate and the Nasal Mucosa.” J Leukoc Biol 80 (2006):797-801.
[Crossref][Google Scholar] [PubMed]
- Ahn, Ji Hoon, Hyunsoo Cho, Jun-Hee Kim, and Shin Heun Kim, et al. “Meningeal Lymphatic Vessels at the Skull Base Drain Cerebrospinal Fluid.” Nature 572 (2019):62-66.
[Crossref][Google Scholar] [PubMed]
- Benveniste, Helene, Hedok Lee, and Nora D. Volkow. “The Glymphatic Pathway: Waste Removal from the CNS via Cerebrospinal Fluid Transport.” Neuroscientist 23 (2017):454-465.
[Crossref][Google Scholar] [PubMed]
- Iliff, Jeffrey J and Maiken Nedergaard. “Is There a Cerebral Lymphatic System?.” Stroke 44 (2013):S93-S95.
[Crossref][Google Scholar] [PubMed]
- Jessen, Nadia Aalling, Anne Sofie Finmann Munk, Iben Lundgaard, and Maiken Nedergaard. “The Glymphatic System: A Beginner's Guide.” Neurochem Res 40 (2015):2583-2599.
[Crossref][Google Scholar] [PubMed]
- Weller, Roy O, Effie Djuanda, Hong-Yeen Yow, and Roxana O Carare. “Lymphatic Drainage of the Brain and the Pathophysiology of Neurological Disease.” Acta Neuropathol 117 (2009):1-14.
[Crossref][Google Scholar] [PubMed]
- Plog, Benjamin A, and Maiken Nedergaard. “The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future.” Annu Rev Pathol 13 (2018):379-394.
[Crossref][Google Scholar] [PubMed]
- Ramirez, Joel, Courtney Berezuk, Alicia A McNeely, and Fuqiang Gao, et al. “Imaging the Perivascular Space as a Potential Biomarker of Neurovascular and Neurodegenerative Diseases.” Cell Mol Neurobiol 36 (2016):289-299.
[Crossref][Google Scholar] [PubMed]
- Johnston, Miles, Dianna Armstrong, and Lena Koh. “Possible Role of the Cavernous Sinus Veins in Cerebrospinal Fluid Absorption.” Cerebrospinal Fluid Res 4 (2007):3.
[Crossref][Google Scholar] [PubMed]
- Tóth, Zsuzsanna E, and Eva Mezey. “Simultaneous Visualization of Multiple Antigens with Tyramide Signal Amplification Using Antibodies from the Same Species.” J Histochem Cytochem 55 (2007):545-554.
[Crossref][Google Scholar] [PubMed]
- Nikolenko, Vladimir N, Marine V Oganesyan, Angela D Vovkogon, and Arina T Nikitina, et al. “Current Understanding of Central Nervous System Drainage Systems: Implications in the Context of Neurodegenerative Diseases.” Curr Neuropharmacol 18 (2020):1054-1063.
[Crossref][Google Scholar] [PubMed]
- Morfoisse, Florent, and Agnès Noel. “Lymphatic and Blood Systems: Identical or Fraternal Twins?.” Int J Biochem Cell Biol 114 (2019):105562.
[Crossref][Google Scholar] [PubMed]
- Oliver, Guillermo. “Lymphatic Vasculature Development.” Nat Rev Immunol 4 (2004):35-45.
[Crossref][Google Scholar] [PubMed]
- Detry, Benoit, Charlotte Erpicum, Jenny Paupert, and Silvia Blacher, et al. “Matrix Metalloproteinase-2 Governs Lymphatic Vessel Formation as an Interstitial Collagenase.” Blood 119 (2012):5048-5056.
[Crossref][Google Scholar] [PubMed]
- Rezaei, Maryam, Mohammad Hashemi, Sara Sanaei, and Mohammad Ali Mashhadi, et al. “Association between Vascular Endothelial Growth Factor Gene Polymorphism.” Breast Cancer (Auckl) 10 (2016):85-91.
[Crossref][Google Scholar] [PubMed]
- Dias, Mariana Castro, Josephine A Mapunda, Mykhailo Vladymyrov, and Britta Engelhardt. “Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers.” Int J Mol Sci 20 (2019):5372.
[Crossref][Google Scholar] [PubMed]
- Ikeshima-Kataoka, Hiroko. “Neuroimmunological Implications of AQP4 in Astrocytes.” Int J Mol Sci 17 (2016):1306.
[Crossref][Google Scholar] [PubMed]
- Jadad, A R, M Moher, Browman GP, and Booker, L et al. “Systematic Reviews and Meta-Analyses on Treatment of Asthma: Critical Evaluation.” BMJ 320 (2000):537-540.
[Crossref][Google Scholar] [PubMed]
- Noé, Francesco M, and Nicola Marchi. “Central Nervous System Lymphatic Unit, Immunity, and Epilepsy: Is There a Link?.” Epilepsia Open 4 (2019):30-39.
[Crossref][Google Scholar] [PubMed]
- Betterman, Kelly L, and Natasha L Harvey. “The Lymphatic Vasculature: Development and Role in Shaping Immunity.” Immunol Rev 271 (2016):276-292.
[Crossref][Google Scholar] [PubMed]
- Von Andrian, Ulrich H, and Thorsten R Mempel. “Homing and Cellular Traffic in Lymph Nodes.” Nat Rev Immunol 3 (2003):867-878.
[Crossref][Google Scholar] [PubMed]
- Amor, Sandra, Fabiola Puentes, David Baker, and Paul van der Valk. "Inflammation in Neurodegenerative Diseases." Immunol 129 (2010):154-169.
- Antila, Salli, Sinem Karaman, Harri Nurmi, and Mikko Airavaara, et al. "Development and Plasticity of Meningeal Lymphatic Vessels." J Exp Med 214 (2017):3645-3667.
[Crossref] [Google Scholar] [Pubmed]
- Abbott, N Joan, and Alon Friedman. "Overview and Introduction: The Blood–Brain Barrier in Health and Disease." Epilepsia 53 (2012):1-6.
[Crossref] [Google Scholar] [Pubmed]
- Marchi, Nicola, Tiziana Granata, Chaitali Ghosh, and Damir Janigro. "Blood–Brain Barrier Dysfunction and Epilepsy: Pathophysiologic Role and Therapeutic Approaches." Epilepsia 53 (2012):1877-1886.
[Crossref] [Google Scholar] [Pubmed]
- Bar-Klein, Guy, Svetlana Lublinsky, Lyn Kamintsky, and Iris Noyman, et al. "Imaging Blood–Brain Barrier Dysfunction as a Biomarker for Epileptogenesis." Brain 140 (2017):1692-1705.
[Crossref] [Google Scholar] [Pubmed]
- Tomkins, Oren, Akiva Feintuch, Moni Benifla, and Avi Cohen, et al. "Blood-Brain Barrier Breakdown Following Traumatic Brain Injury: A Possible Role in Posttraumatic Epilepsy." Cardiovasc Psychiatry Neurol 2011 (2011):765923
[Crossref] [Google Scholar] [Pubmed]
- Frigerio, Federica, Angelisa Frasca, Itai Weissberg, and Sara Parrella, et al. "Long-Lasting Pro-Ictogenic Effects Induced In Vivo by Rat Brain Exposure to Serum Albumin in the Absence of Concomitant Pathology." Epilepsia 53 (2012):1887-1897.
[Crossref] [Google Scholar] [Pubmed]
- Marchi, Nicola, Tiziana Granata, and Damir Janigro. "Inflammatory Pathways of Seizure Disorders." Trends Neurosci 37 (2014):55-65.
[Crossref] [Google Scholar] [Pubmed]
- Vezzani, Annamaria, Bethan Lang, and Eleonora Aronica. "Immunity and Inflammation in Epilepsy." Cold Spring Har Perspect Med 6 (2016):a022699.
[Crossref] [Google Scholar] [Pubmed]
- Vazana, Udi, Ronel Veksler, Gaby S Pell, and Ofer Prager, et al. "Glutamate-Mediated Blood–Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery." J Neurosci 36 (2016):7727-7739.
[Crossref] [Google Scholar] [Pubmed]
- Klement, Wendy, Rita Garbelli, Emma Zub, and Laura Rossini, et al. "Seizure Progression and Inflammatory Mediators Promote Pericytosis and Pericyte-Microglia Clustering at the Cerebrovasculature." Neurobiol Dis 113 (2018):70-81.
[Crossref] [Google Scholar] [Pubmed]
- Engel, Tobias, Paloma Goñi-Oliver, José J Lucas, and Jesús Avila, et al. "Chronic Lithium Administration to FTDP-17 Tau and GSK-3ß Overexpressing Mice Prevents Tau Hyperphosphorylation and Neurofibrillary Tangle Formation, But Pre-Formed Neurofibrillary Tangles Do Not Revert." J Neurochem 99 (2006):1445-1455.
[Crossref] [Google Scholar] [Pubmed]
- Foyaca-Sibat, Humberto, Salazar-Campos M, and Ibañez-Valdés L. “Cysticercosis of the Extraocular Muscles. Our Experience and Review of the Medical Literature.” Inte J of Neurol 14 (2012).
- Foyaca-Sibat, Humberto, and Ibañez-Valdés L. “Introduction to Cysticercosis and its Historical Background.” Nov Aspec Cysticerco Neurocystic (2013).
- Foyaca-Sibat, Humberto, and Ibañez-Valdés L. “What is a Low Frequency of the Disseminated Cysticercosis Suggests that Neurocysticercosis is Going to Disappear?.” Nov Aspec Cysticerco Neurocystic (2013).
- Foyaca-Sibat, Humberto, and Ibañez-Valdés L. “Uncommon Clinical Manifestations of Cysticercosis.” Nov Aspec Cysticerco Neurocystic (2013).
- Ibañez-Valdés, Lourdes de Fátima, and Humberto Foyaca-Sibat. “Psychogenic Nonepileptic Seizures in Patients Living with Neurocysticercosis.” Seizures (2018).
- Foyaca-Sibat, Humberto, and Ibañez-Valdés L. “Subarachnoid Cysticercosis and Ischaemic Stroke in Epileptic Patients.” Seizures (2017).
- Noormahomed, Emilia Virgínia, Noémia N, Jerónimo M, and Robert TS, et al. “Neurocysticercosis in Epileptic Children: An Overlooked Condition in Mozambique, Challenges in Diagnosis, Management and Research Priorities.” EC Microbiology 17 (2021):49-56.
- Foyaca-Sibat, Humberto. “Racemose Neurocysticercosis Long COVID and Brainstem Dysfunction: A Case report and Systematic Review.” Clin Schizophr Relat Psychoses 15S (2021).
- Foyaca-Sibat, Humberto. “Neurocysticercosis, Epilepsy, COVID-19 and a Novel Hypothesis: Cases Series and Systematic Review.” Clin Schizophr Relat Psychoses 15S (2021):1-13
- Foyaca-Sibat, Humberto. “People Living with HIV and Neurocysticercosis Presenting Covid-19: A Systematic Review and Crosstalk Proposals.” Clin Schizophr Relat Psychoses 15S (2021):1-9
- Foyaca-Sibat, Humberto. “Comorbidity of Neurocysticercosis, HIV, Cerebellar Atrophy and SARS-CoV-2: Case Report and Systematic Review.” Clin Schizophr Relat Psychoses 15S (2021):1-6.
- Cueni, Leah N, and Michael Detmar. “The Lymphatic System in Health and Disease.” Lymphat Res Biol 6 (2008):109-122.
[Crossref][Google Scholar] [PubMed]
- Tamura, Ryota, Kazunari Yoshida, and Masahiro Toda. “Current Understanding of Lymphatic Vessels in the Central Nervous System.” Neurosurg Rev 43 (2020):1055-1064.
[Crossref][Google Scholar] [PubMed]
- Johnston, Miles, Andrei Zakharov, Christina Papaiconomou, and Giselle Salmasi, et al. “Evidence of Connections between Cerebrospinal Fluid and Nasal Lymphatic Vessels in Humans, Non-Human Primates and Other Mammalian Species.” Cerebrospinal Fluid Res 1 (2004):2.
[Crossref][Google Scholar] [PubMed]
- Wang, H J, and J R Casley-Smith. “Drainage of the Prelymphatics of the Brain via the Adventitia of the Vertebral Artery.” Acta Anat (Basel) 134 (1989):67-71.
[Crossref][Google Scholar] [PubMed]
- Li, J, J Zhou, and Y Shi. “Scanning Electron Microscopy of Human Cerebral Meningeal Stomata.” Ann Anat 178 (1996):259-261.
[Crossref][Google Scholar] [PubMed]
- Gao, Yu-fei, and Zhan-quan Yang. “Experimental Study of Cranial-Cervical Lymph Return in Rabbit.” Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 40 (2005):182-185.
[Google Scholar] [PubMed]
- Johnston, Miles, Andrei Zakharov, Christina Papaiconomou, and Giselle Salmasi. “Evidence of Connections between Cerebrospinal Fluid and Nasal Lymphatic Vessels in Humans, Non-Human Primates and Other Mammalian Species.” Cerebrospinal Fluid Res 1 (2004):2.
[Crossref][Google Scholar] [PubMed]
- Foyaca-Sibat, Humberto. “Intracranial Hypotension after Severe COVID-19: Case Report and Literature Review.” Clin Schizophr Relat Psychoses 15S (2021):1-11
- Marín-Padilla, Miguel, and David S Knopman. “Developmental Aspects of the Intracerebral Microvasculature and Perivascular Spaces: Insights into Brain Response to Late-Life Diseases.” J Neuropathol Exp Neurol 70 (2011):1060-1069.
[Crossref][Google Scholar] [PubMed]
- Begley, David J. “Brain Superhighways.” Sci Transl Med 4 (2012):147fs29.
[Crossref][Google Scholar] [PubMed]
- Iliff, Jeffrey J, Minghuan Wang, Yonghong Liao, and Benjamin A Plogg, et al. “A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid ß.” Sci Transl Med 4 (2012):147ra111.
[Crossref][Google Scholar] [PubMed]
- Xie, Lulu, Hongyi Kang, Qiwu Xu, and Michael J Chen, et al. “Sleep Drives Metabolite Clearance from the Adult Brain.” Science 342 (2013):373-377.
[Crossref][Google Scholar] [PubMed]
- Weller, Roy O, Effie Djuanda, Hong-Yeen Yow, and Roxana O Carare. “Lymphatic Drainage of the Brain and the Pathophysiology of Neurological Disease.” Acta Neuropathol 117 (2009):1-14.
[Crossref][Google Scholar] [PubMed]
- Williams, K, X Alvarez, and Lackner AA. “Central Nervous System Perivascular Cells are Immunoregulatory Cells that Connect the CNS with the Peripheral Immune System.” Glia 36 (2001):156-164.
[Crossref][Google Scholar] [PubMed]
- Bechmann, Ingo, Ian Galea, and Hugh Perry V. “What is the Blood-Brain Barrier (Not)?.” Trends Immunol 28 (2007):5-11.
[Crossref][Google Scholar] [PubMed]
- Galea, Ian, Ingo Bechmann, and Hugh Perry V. “What is Immune Privilege (Not)?.” Trends Immunol 28 (2007):12-18.
[Crossref][Google Scholar] [PubMed]
- Schaik, Caroline J van, Lucas L Boer, Jos M T Draaisma, and Carine J M van der Vleuten, et al. “The Lymphatic System throughout History: From Hieroglyphic Translations to State of the Art Radiological Techniques.” Clin Anat 35 (2022):701-710.
[Crossref][Google Scholar] [PubMed]
- Catola, G, and N Achúcarro. “Über die Entstehung der Amyloidkörperchen im Zentralnervensystem.” Virchows Arch f Path Anat 184 (1906):454-459.
- Virchow, Rud. “Ueber eine im Gehirn und Rückenmark des Menschen Aufgefundene Substanz mit der Chemischen Reaction der Cellulose.” Arch Pathol Anat Physiol Klin Med 6 (1854):135-138.
- Schwalbe, G. “Die Arachnoidalraum ein Lymphraum und sein Zusammenhang mit den Perichorioidalraum.” Zbl Med Wiss. 7 (1869):465-467.
- Key, A, and Retzius G. “Studien in der Anatomie des Nervensystems und des Bindegewebes.” Samson and Wallin 1 (1875)
- Bruce, A, and Dawson, JW. “On the Relations of the Lymphatics of the Spinal Cord.” J Pathol Bacteriol 15 (1911):169-178.
- Obersteiner, H. “The Anatomy of the Central Nervous Organs in Health and Disease.” Griffin (1900):174-175.
- Andres, K H, Düring M von, Muszynski K, and Schmidt RF. “Nerve Fibres and Their Terminals of the Dura Mater Encephali of the Rat.” Anat Embryol (Berl) 175 (1987):289-301.
[Crossref][Google Scholar] [PubMed]
- Aspelund, Aleksanteri, Salli Antila, Steven T Proulx, and Tine Veronica Karlsen, et al. “A Dural Lymphatic Vascular System That Drains Brain Interstitial Fluid and Macromolecules.” J Exp Med 212 (2015):991-999.
[Crossref][Google Scholar] [PubMed]
- Louveau, Antoine, Igor Smirnov, Timothy J Keyes, and Jacob D Eccles, et al. “Structural and Functional Features of Central Nervous System Lymphatic Vessels.” Nature 523 (2015):337-341.
[Crossref][Google Scholar] [PubMed]
- Cserr, H F, Harling-Berg CJ, and Knopf PM. “Drainage of Brain Extracellular Fluid into Blood and Deep Cervical Lymph and Its Immunological Significance.” Brain Pathol 2 (1992):269-276.
[Crossref][Google Scholar] [PubMed]
- Sobel, RA, Mitchell ME, and Fondren G. “Intercellular Adhesion Molecule-1 (ICAM-1) in Cellular Immune Reactions in the Human Central Nervous System.” Am J Pathol 136 (1990):1309-1316.
[Google Scholar] [PubMed]
- Engelhardt, Britta, and Richard M Ransohoff. “The Ins and Outs of T-Lymphocyte Trafficking to the CNS: Anatomical Sites and Molecular Mechanisms.” Trends Immunol 26 (2005):485-495.
[Crossref][Google Scholar] [PubMed]
- Lackie, JM. “A Dictionary of Biomedicine.” Oxford University Press (2010).
- Sen, Arjune, Maria Thom, Lillian Martinian, and Brian Harding, et al. "Pathological Tau Tangles Localize To Focal Cortical Dysplasia In Older Patients." Epilepsia 48 (2007):1447-1454.
[Crossref] [Google Scholar] [Pubmed]
- Liang, Zhihou, Fei Liu, Khalid Iqbal, and Inge Grundke-Iqbal, et al. "Dysregulation of Tau Phosphorylation in Mouse Brain during Excitotoxic Damage." J Alzheimers Dis 17 (2009):531-539.
[Crossref] [Google Scholar] [Pubmed]
- Roberson, Erik D, Brian Halabisky, Jong W Yoo, and Jinghua Yao, et al. "Amyloid-ß/Fyn–Induced Synaptic, Network, and Cognitive Impairments Depend on Tau Levels in Multiple Mouse Models of Alzheimer's Disease." J Neurosci 31 (2011):700-711.
[Crossref] [Google Scholar] [Pubmed]
- Thom, Maria, Joan YW Liu, Pam Thompson, and Rahul Phadke, et al. "Neurofibrillary Tangle Pathology and Braak Staging in Chronic Epilepsy in Relation to Traumatic Brain Injury and Hippocampal Sclerosis: A Post-Mortem Study." Brain 134 (2011):2969-2981.
[Crossref] [Google Scholar] [Pubmed]
- Jones, Nigel C, Thanh Nguyen, Niall M Corcoran, and Dennis Velakoulis, et al. "Targeting Hyperphosphorylated Tau with Sodium Selenate Suppresses Seizures in Rodent Models." Neurobiol Dis 45 (2012):897-901.
[Crossref] [Google Scholar] [Pubmed]
- Hawkins, Bridget E, Shashirekha Krishnamurthy, Diana L Castillo-Carranza, and Urmi Sengupta, et al. "Rapid Accumulation of Endogenous Tau Oligomers in a Rat Model of Traumatic Brain Injury: Possible Link between Traumatic Brain Injury and Sporadic Tauopathies." J Biol Chem 288 (2013):17042-17050.
[Crossref] [Google Scholar] [Pubmed]
- Kandratavicius, Ludmyla, Mariana Raquel Monteiro, Jaime Eduardo Hallak, and Carlos Gilberto Carlotti, et al. "Microtubule-Associated Proteins in Mesial Temporal Lobe Epilepsy with and Without Psychiatric Comorbidities and their Relation with Granular Cell Layer Dispersion." Biomed Res Int 2013 (2013):960126
[Crossref] [Google Scholar] [Pubmed]
- Zheng, Ping, Sandy R Shultz, Chris M Hovens, and Dennis Velakoulis, et al. "Hyperphosphorylated Tau is Implicated in Acquired Epilepsy and Neuropsychiatric Comorbidities." Mol Neurobiol 49 (2014):1532-1539.
[Crossref] [Google Scholar] [Pubmed]
- Sarnat, Harvey B, and Laura Flores-Sarnat. "Infantile Tauopathies: Hemimegalencephaly; Tuberous Sclerosis Complex; Focal Cortical Dysplasia 2; Ganglioglioma." Brain Dev 37 (2015):553-562.
[Crossref] [Google Scholar] [Pubmed]
- Shultz, Sandy R, David K Wright, Ping Zheng, and Ryan Stuchbery, et al. "Sodium Selenate Reduces Hyperphosphorylated Tau and Improves Outcomes after Traumatic Brain Injury." Brain 138 (2015):1297-1313.
[Crossref] [Google Scholar] [Pubmed]
- Monti, Giulia, Manuela Tondelli, Giada Giovannini, and Roberta Bedin, et al. "Cerebrospinal Fluid Tau Proteins in Status Epilepticus." Epilepsy Behav 49 (2015):150-154.
[Crossref] [Google Scholar] [Pubmed]
- Puvenna, Vikram, Madeline Engeler, Manoj Banjara, and Chanda Brennan, et al. "Is Phosphorylated Tau Unique to Chronic Traumatic Encephalopathy? Phosphorylated Tau in Epileptic Brain and Chronic Traumatic Encephalopathy." Brain Res 1630 (2016):225-240.
[Crossref] [Google Scholar] [Pubmed]
- Decker, Jochen Martin, Lars Krüger, Astrid Sydow, and Frank JA Dennissen, et al. "The Tau/A152T Mutation, A Risk Factor for Frontotemporal-Spectrum Disorders, Leads to NR 2B Receptor-Mediated Excitotoxicity." EMBO Rep 17 (2016):552-569.
[Crossref] [Google Scholar] [Pubmed]
- Liu, Shi-jie, Ping Zheng, David K Wright, and Gabi Dezsi, et al. "Sodium Selenate Retards Epileptogenesis in Acquired Epilepsy Models Reversing Changes in Protein Phosphatase 2A and Hyperphosphorylated Tau." Brain 139 (2016):1919-1938.
[Crossref] [Google Scholar] [Pubmed]
- Sotiropoulos, Ioannis, Marie-Christine Galas, Joana M Silva, and Efthimios Skoulakis, et al. "Atypical, Non-Standard Functions of the Microtubule Associated Tau Protein." Acta Neuropathol Commun 5 (2017):1-11.
[Crossref] [Google Scholar] [Pubmed]
- Wilson, Lindsay, William Stewart, Kristen Dams-O'Connor, and Ramon Diaz-Arrastia, et al. "The Chronic and Evolving Neurological Consequences of Traumatic Brain Injury." Lancet Neurol 16 (2017):813-825.
[Crossref] [Google Scholar] [Pubmed]
- Bauer, Jan, Albert J Becker, Wassim Elyaman, and Jukka Peltola, et al. "Innate and Adaptive Immunity in Human Epilepsies." Epilepsia 58 (2017):57-68.
[Crossref] [Google Scholar] [Pubmed]
- Lancaster, Eric. "The Diagnosis and Treatment of Autoimmune Encephalitis." J Clin Neurol 12 (2016):1-13.
[Crossref] [Google Scholar] [Pubmed]
- Bauer, Jan, Annamaria Vezzani, and Christian G Bien. "Epileptic Encephalitis: The Role of the Innate and Adaptive Immune System." Brain Pathol 22 (2012):412-421.
[Crossref] [Google Scholar] [Pubmed]
- Haraldsen, Guttorm, Dag Kvale, Bjorn Lien, and Inger N Farstad, et al. "Cytokine-Regulated Expression of E-Selectin, Intercellular Adhesion Molecule-1 (ICAM-1), and Vascular Cell Adhesion Molecule-1 (VCAM-1) in Human Microvascular Endothelial Cells." J Immunol 156 (1996):2558-2565.
[Crossref] [Google Scholar] [Pubmed]
- Karaman, Sinem, Harri Nurmi, Salli Antila, and Kari Alitalo. "Stimulation and Inhibition of Lymphangiogenesis via Adeno-Associated Viral Gene Delivery." Methods Mol Biol (2018):291-300.
[Crossref] [Google Scholar] [Pubmed]
- Beattie, Eric C, David Stellwagen, Wade Morishita, and Jacqueline C Bresnahan, et al. "Control of Synaptic Strength by Glial TNFa." Science 295 (2002):2282-2285.
[Crossref] [Google Scholar] [Pubmed]
- Golan, Hava, T Levav, A Mendelsohn, and M Huleihel. "Involvement of Tumor Necrosis Factor Alpha in Hippocampal Development and Function." Cereb Cortex 14 (2004):97-105.
[Crossref] [Google Scholar] [Pubmed]
- Stellwagen, David, Eric C Beattie, Jae Y Seo, and Robert C Malenka. "Differential Regulation of AMPA Receptor and GABA Receptor Trafficking by Tumor Necrosis Factor-a." J Neurosci 25 (2005):3219-3228.
[Crossref] [Google Scholar] [Pubmed]
- Stellwagen, David, and Robert C Malenka. "Synaptic Scaling Mediated by Glial TNF-a." Nature 440 (2006):1054-1059.
[Crossref] [Google Scholar] [Pubmed]
- Iosif, Robert E, Christine T Ekdahl, Henrik Ahlenius, and Cornelis JH Pronk, et al. "Tumor Necrosis Factor Receptor 1 is a Negative Regulator of Progenitor Proliferation in Adult Hippocampal Neurogenesis." J Neurosci 26 (2006):9703-9712.
[Crossref] [Google Scholar] [Pubmed]
- Baune, Bernhard T, Florian Wiede, Anja Braun, and Jonathan Golledge, et al. "Cognitive Dysfunction in Mice Deficient for TNF-and its Receptors." Am J Med Genet B Neuropsychiatr Genet 147 (2008):1056-1064.
[Crossref] [Google Scholar] [Pubmed]
- Chen, Zhiguo, and Theo D Palmer. "Differential Roles of TNFR1 and TNFR2 Signaling in Adult Hippocampal Neurogenesis." Brain Behav Immun 30 (2013):45-53.
[Crossref] [Google Scholar] [Pubmed]
- Dellarole, Anna, Paul Morton, Roberta Brambilla, and Winston Walters, et al. "Neuropathic Pain-Induced Depressive-Like Behavior and Hippocampal Neurogenesis and Plasticity are Dependent on TNFR1 Signaling." Brain Behav Immun 41 (2014):65-81.
[Crossref] [Google Scholar] [Pubmed]
- Liu, Bingfang, Bojana Zupan, Emma Laird, and Shifra Klein, et al. "Maternal Hematopoietic TNF, via Milk Chemokines, Programs Hippocampal Development and Memory." Nat Neurosci 17 (2014):97-105.
[Crossref] [Google Scholar] [Pubmed]
- Cheadle, Lucas, Christopher P Tzeng, Brian T Kalish, and David A Harmin, et al. "Visual Experience-Dependent Expression of Fn14 is Required for Retinogeniculate Refinement." Neuron 99 (2018):525-539.
[Crossref] [Google Scholar] [Pubmed]
- Davalos, Dimitrios, Jaime Grutzendler, Guang Yang, and Jiyun V Kim, et al. "ATP Mediates Rapid Microglial Response to Local Brain Injury In Vivo." Nat Neurosci 8 (2005):752-758.
[Crossref] [Google Scholar] [Pubmed]
- Nimmerjahn, Axel, Frank Kirchhoff, and Fritjof Helmchen. "Resting Microglial Cells are Highly Dynamic Surveillants of Brain Parenchyma In Vivo." Science 308 (2005):1314-1318.
[Crossref] [Google Scholar] [Pubmed]
- Ginhoux, Florent, Melanie Greter, Marylene Leboeuf, and Sayan Nandi, et al. "Fate Mapping Analysis Reveals that Adult Microglia Derive from Primitive Macrophages." Science 330 (2010):841-845.
[Crossref] [Google Scholar] [Pubmed]
- Paolicelli, Rosa C, Giulia Bolasco, Francesca Pagani, and Laura Maggi, et al. "Synaptic Pruning by Microglia is Necessary for Normal Brain Development." Science 333 (2011):1456-1458.
[Crossref] [Google Scholar] [Pubmed]
- Schafer, Dorothy P, Emily K Lehrman, Amanda G Kautzman, and Ryuta Koyama, et al. "Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner." Neuron 74 (2012):691-705.
[Crossref] [Google Scholar] [Pubmed]
- Parkhurst, Christopher N, Guang Yang, Ipe Ninan, and Jeffrey N Savas, et al. "Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor." Cell 155 (2013):1596-1609.
[Crossref] [Google Scholar] [Pubmed]
- Lavin, Yonit, Deborah Winter, Ronnie Blecher-Gonen, and Eyal David, et al. "Tissue-Resident Macrophage Enhancer Landscapes are Shaped by the Local Microenvironment." Cell 159 (2014):1312-1326.
[Crossref] [Google Scholar] [Pubmed]
- Gosselin, David, Verena M Link, Casey E Romanoski, and Gregory J Fonseca, et al. "Environment Drives Selection and Function of Enhancers Controlling Tissue-Specific Macrophage Identities." Cell 159 (2014):1327-1340.
[Crossref] [Google Scholar] [Pubmed]
- Butovsky, Oleg, Mark P Jedrychowski, Craig S Moore, and Ron Cialic, et al. "Identification of a Unique TGF-ß–Dependent Molecular and Functional Signature in Microglia." Nat Neurosci 17 (2014):131-143.
[Crossref] [Google Scholar] [Pubmed]
- Gomez Perdiguero, Elisa, Kay Klapproth, Christian Schulz, and Katrin Busch, et al. "Tissue-Resident Macrophages Originate From Yolk-Sac-Derived Erythro-Myeloid Progenitors." Nature 518 (2015):547-551.
[Crossref] [Google Scholar] [Pubmed]
- Schafer, Dorothy P, Christopher T Heller, Georgia Gunner, and Molly Heller, et al. "Microglia Contribute to Circuit Defects in Mecp2 Null Mice Independent of Microglia-Specific Loss of Mecp2 Expression." Elife 5 (2016):e15224.
[Crossref] [Google Scholar] [Pubmed]
- Matcovitch-Natan, Orit, Deborah R Winter, Amir Giladi, and Stephanie Vargas Aguilar, et al. "Microglia Development Follows a Stepwise Program to Regulate Brain Homeostasis." Science 353 (2016):aad8670.
[Crossref] [Google Scholar] [Pubmed]
- Hong, Soyon, Victoria F Beja-Glasser, Bianca M Nfonoyim, and Arnaud Frouin, et al. "Complement and Microglia Mediate Early Synapse Loss in Alzheimer Mouse Models." Science 352 (2016):712-716.
[Crossref] [Google Scholar] [Pubmed]
- Vasek, Michael J, Charise Garber, Denise Dorsey, and Douglas M Durrant, et al. "A Complement–Microglial Axis Drives Synapse Loss During Virus-Induced Memory Impairment." Nature 534 (2016):538-543.
[Crossref] [Google Scholar] [Pubmed]
- Fourgeaud, Lawrence, Paqui G Través, Yusuf Tufail, and Humberto Leal-Bailey, et al. "TAM Receptors Regulate Multiple Features of Microglial Physiology." Nature 532 (2016):240-244.
[Crossref] [Google Scholar] [Pubmed]
- Liddelow, Shane A, Kevin A Guttenplan, Laura E Clarke, and Frederick C Bennett, et al. "Neurotoxic Reactive Astrocytes are Induced by Activated Microglia." Nature 541 (2017):481-487.
[Crossref] [Google Scholar] [Pubmed]
- Réu, Pedro, Azadeh Khosravi, Samuel Bernard, and Jeff E Mold, et al. "The Lifespan and Turnover of Microglia in the Human Brain." Cell Rep 20 (2017):779-784.
[Crossref] [Google Scholar] [Pubmed]
- Gosselin, David, Dylan Skola, Nicole G Coufal, and Inge R Holtman, et al. "An Environment-Dependent Transcriptional Network Specifies Human Microglia Identity." Science 356 (2017):eaal3222.
[Crossref] [Google Scholar] [Pubmed]
- Hagemeyer, Nora, Klara-Maria Hanft, Maria-Anna Akriditou, and Nicole Unger, et al. "Microglia Contribute to Normal Myelinogenesis and to Oligodendrocyte Progenitor Maintenance during Adulthood." Acta Neuropathol 134 (2017):441-458.
[Crossref] [Google Scholar] [Pubmed]
- Wlodarczyk, Agnieszka, Inge R Holtman, Martin Krueger, and Nir Yogev, et al. "A Novel Microglial Subset Plays a Key Role in Myelinogenesis in Developing Brain." EMBO J 36 (2017):3292-3308.
[Crossref] [Google Scholar] [Pubmed]
- Bennett, F Chris, Mariko L Bennett, Fazeela Yaqoob, and Sara B Mulinyawe, et al. "A Combination of Ontogeny and CNS Environment Establishes Microglial Identity." Neuron 98 (2018):1170-1183.
[Crossref] [Google Scholar] [Pubmed]
- Li, Qingyun, and Ben A Barres. "Microglia and Macrophages in Brain Homeostasis and Disease." Nat Rev Immunol 18 (2018):225-242.
[Crossref] [Google Scholar] [Pubmed]
- Lund, Harald, Melanie Pieber, Roham Parsa, and David Grommisch, et al. "Fatal Demyelinating Disease is induced by Monocyte-derived Macrophages in the Absence of TGF-ß Signaling." Nat Immunol 19, (2018):1-7.
[Crossref] [Google Scholar] [Pubmed]
- Weinhard, Laetitia, Giulia Di Bartolomei, Giulia Bolasco, and Pedro Machado, et al. "Microglia Remodel Synapses by Presynaptic Trogocytosis and Spine Head Filopodia Induction." Nat Commun 9 (2018):1228.
[Crossref] [Google Scholar] [Pubmed]
- Li, Qingyun, Zuolin Cheng, Lu Zhou, and Spyros Darmanis, et al. "Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing." Neuron 101 (2019):207-223.
[Crossref] [Google Scholar] [Pubmed]
- Hammond, Timothy R, Connor Dufort, Lasse Dissing-Olesen, and Stefanie Giera, et al. "Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes." Immunity 50 (2019):253-271.
[Crossref] [Google Scholar] [Pubmed]
- Kitchen, Philip, Rebecca E Day, Mootaz M Salman, and Matthew T Conner, et al. "Beyond Water Homeostasis: Diverse Functional Roles of Mammalian Aquaporins." Biochim Biophys Acta 1850 (2015):2410-2421.
[Crossref] [Google Scholar] [Pubmed]
- Liu, Kewei, Juan Zhu, Yuan Chang, and Zhenzhou Lin, et al. "Attenuation of Cerebral Edema Facilitates Recovery of Glymphatic System Function after Status Epilepticus." JCI Insight 6 (2021):e151835.
[Crossref] [Google Scholar] [Pubmed]
- Hasan-Olive, Md Mahdi, Rune Enger, Hans-Arne Hansson, and Erlend A Nagelhus, et al. "Loss of Perivascular Aquaporin-4 in Idiopathic Normal Pressure Hydrocephalus." Glia 67 (2019):91-100.
[Crossref] [Google Scholar] [Pubmed]
- Reeves, Benjamin C, Jason K Karimy, Adam J Kundishora, and Humberto Mestre, et al. "Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus." Trends Mol Med 26 (2020):285-295.
[Crossref] [Google Scholar] [Pubmed]
- Ivan, Daniela C, Sabrina Walthert, Kristina Berve, and Jasmin Steudler, et al. "Dwellers and Trespassers: Mononuclear Phagocytes at the Borders of the Central Nervous System." Front Immunol 11 (2021):609921.
[Crossref] [Google Scholar] [Pubmed]
- Kitchen, Philip, Mootaz M Salman, Andrea M Halsey, and Charlotte Clarke-Bland, et al. "Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema." Cell 181 (2020):784-799.
[Crossref] [Google Scholar] [Pubmed]
- Morfoisse, Florent, and Agnes Noel. "Lymphatic and Blood Systems: Identical or Fraternal Twins?." Int J Biochem Cell Biol 114 (2019):105562.
[Crossref] [Google Scholar] [Pubmed]
- Mogensen, Frida Lind-Holm, Christine Delle, and Maiken Nedergaard. "The Glymphatic System (En) During Inflammation." Int J Mol Sci 22 (2021):7491.
[Crossref] [Google Scholar] [Pubmed]
- Xie, Lulu, Hongyi Kang, Qiwu Xu, and Michael J Chen, et al. "Sleep Drives Metabolite Clearance from the Adult Brain." Science 342 (2013):373-377.
[Crossref] [Google Scholar] [Pubmed]
- Borbély, Alexander A, Serge Daan, Anna Wirz-Justice, and Tom Deboer. "The Two-Process Model of Sleep Regulation: A Reappraisal." J Sleep Res 25 (2016):131-143.
[Crossref] [Google Scholar] [Pubmed]
- Schain, Aaron J, Agustin Melo-Carrillo, Andrew M Strassman, and Rami Burstein. "Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache." J Neurosci 37 (2017):2904-2915.
[Crossref] [Google Scholar] [Pubmed]
- Fultz, Nina E, Giorgio Bonmassar, Kawin Setsompop, and Robert A Stickgold, et al. "Coupled Electrophysiological, Hemodynamic, and Cerebrospinal Fluid Oscillations in Human Sleep." Science 366 (2019):628-631.
[Crossref] [Google Scholar] [Pubmed]
- Harrison, Ian F, Ozama Ismail, Asif Machhada, and Niall Colgan, et al. "Impaired Glymphatic Function and Clearance of Tau in an Alzheimer’s Disease Model." Brain 143 (2020):2576-2593.
[Crossref] [Google Scholar] [Pubmed]
- Nedergaard, Maiken, and Steven A Goldman. "Glymphatic Failure as a Final Common Pathway to Dementia." Science 370 (2020):50-56.
[Crossref] [Google Scholar] [Pubmed]
- Christensen, Jennaya, Glenn R Yamakawa, Sandy R Shultz, and Richelle Mychasiuk. "Is the Glymphatic System the Missing Link Between Sleep Impairments and Neurological Disorders? Examining the Implications and Uncertainties." Progr Neurobiol 198 (2021):101917.
[Crossref] [Google Scholar] [Pubmed]
- Demiral, Sükrü Baris, Dardo Tomasi, Joelle Sarlls, and Hedok Lee, et al. "Apparent Diffusion Coefficient Changes in Human Brain during Sleep–Does it Inform on the Existence of a Glymphatic System?." Neuroimage 185 (2019):263-273.
[Crossref] [Google Scholar] [Pubmed]
- Elvsåshagen, Torbjørn, Henri Jmm Mutsaerts, Nathalia Zak, and Linn B Norbom, et al. "Cerebral Blood Flow Changes after a day of Wake, Sleep, and Sleep Deprivation." Neuroimage 186 (2019):497-509.
[Crossref] [Google Scholar] [Pubmed]
- Bernardi, Giulio, Luca Cecchetti, Francesca Siclari, and Andreas Buchmann, et al. "Sleep Reverts Changes in Human Gray and White Matter caused by Wake-Dependent Training." Neuroimage 129 (2016):367-377.
[Crossref] [Google Scholar] [Pubmed]
- Kim, Young-Kook, Kwang Il Nam, and Juhyun Song. "The Glymphatic System in Diabetes-induced Dementia." Front Neurol 9 (2018):867.
[Crossref] [Google Scholar] [Pubmed]
- Iliff, Jeffrey J, Steven A Goldman, and Maiken Nedergaard. "Implications of the Discovery of Brain Lymphatic Pathways." Lancet Neurol 14 (2015):977-979.
[Crossref] [Google Scholar] [Pubmed]
- Zhang, Rui, Yun Liu, Yan Chen, and Qian Li, et al. "Aquaporin 4 Deletion Exacerbates Brain Impairments in a Mouse Model of Chronic Sleep Disruption." CNS Neurosci Ther 26 (2020):228-239.
[Crossref] [Google Scholar] [Pubmed]
- Li, Wenzhong, Dawei Chen, Nan Liu, and Yongxin Luan, et al. "Modulation of Lymphatic Transport in the Central Nervous System." Theranostics 12 (2022):1117-1131.
- Somers, Virend K, Mark E Dyken, Allyn L Mark, and Francois M Abboud. "Sympathetic-Nerve Activity during Sleep in Normal Subjects." N Engl J Med 328 (1993):303-307.
[Crossref] [Google Scholar] [Pubmed]
- Brown, Ritchie E, Radhika Basheer, James T McKenna, and Robert E Strecker, et al. "Control of Sleep and Wakefulness." Physiol Rev 92 (2012):1087-1187.
- Park, Jin Won, Hyun Woo Chung, Eun Jeong Lee, and Kyung-Ho Jung, et al. "a2-Adrenergic Agonists including Xylazine and Dexmedetomidine inhibit Norepinephrine Transporter Function in SK-N-SH Cells." Neurosci Lett 541 (2013):184-189.
[Crossref] [Google Scholar] [Pubmed]
- Riba, Marta, Elisabet Augé, Joan Campo-Sabariz, and David Moral-Anter, et al. "Corpora Amylacea Act as Containers that Remove Waste Products from the Brain." Proc Natl Acad Sci 116 (2019):26038-26048.
[Crossref] [Google Scholar] [Pubmed]
Citation: Valdes , Lourdes Fatima de Ibanez and Humberto "Meningeal Lymphatic Vessels and GLymphatic System in Neurocysticercosis: A Systematic Review and Novel Hypotheses." Clin Schizophr Relat Psychoses 17 (2023).
Copyright: © 2023 Sibat HF, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.