Research Article - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Inflammatory Associations Of Peripheral Oxytocin Creactive Protein Levels With Depression Among Adult Age Group With Maj
Amer Fadhil Alhaideri1, Azher Nema Mohammed Al-Agam2, Hayder Abdul-Amir M Al-Hindy3*, Mazin J Mousa3, Hawraa Kadhum3 and Saja Hatem32Department of Psychiatry, Merjan Teaching Hospital, Babylon, Iraq
3Department of Pharmacy, University of Babylon, Babylon, Iraq
Hayder Abdul-Amir M Al-Hindy, Department of Pharmacy, University of Babylon, Babylon, Iraq, Email: phar.hayder.abdul@uobabylon.edu.iq
Received: 01-Oct-2021 Accepted Date: Oct 15, 2021 ; Published: 22-Oct-2021
Abstract
Background: Major Depressive Disorder (MDD) is a common mental illness. Even though MDD is not exactly an inflammatory disorder, inflammation displays a substantial input, which may even predict the new onset of depressive thoughts. As a "nonspecific acute phase reactant" synthesized in the liver cells as an inflammatory response, C-reactive protein (CRP) is a marker of "low-grade inflammation as noted by overwhelming shreds of evidence. Some scientists assume a likely neuro immune psychological interaction between negative affect (depressive attitude, anger, anxiety, and poor prosperity), and inflammatory responses. Oxytocin a neuropeptide hormone has wide physiological effects, the ability to support health, and impact behavior as revealed by the growing evidence for its actions through the immune response and as an anti-inflammatory issue. The data concerning the influence of oxytocin hormone in MDD is somewhat limited.
Our aim in this work was to assess the inflammatory associations of peripheral oxytocin and CRP levels in depression, among the adult age group with MDD.
Materials and methods: This observational study had included 180 patients recognized as MDD after their fulfillment of "DSM-5 criteria for MDD version 7.0.2", using the "Mini International Neuropsychiatric Interview". A self-administered form had applied for evaluating the severity of depression according to the criteria of MDD by using a 9-items questionnaire based on the "Patient Health Questionnaire depression module (PHQ-9)". BMI calculation and biochemical assays of serum levels of oxytocin and CRP had been achieved for all participants. Variations in demographic variables were calculated using t-tests for continuous variables and Pearson correlations had used to judge associations between the parameters. ROC curve had used to inspect the predictive ability of both CRP and oxytocin to diagnose severe depressive symptoms.
Results: The mean serum CRP levels were relatively high (9.0 ± 9.2 mg/ml) among MDD patients, whereas the mean oxytocin was 33.5 pg/ml. There were nonsignificant alterations between sexes in all study parameters other than oxytocin, which was higher among females. No significant disparities in the distribution of both oxytocin and CRP serum values among the scores of PHQ-9. A significant difference in the mean levels of serum CRP but not oxytocin between those having mild and severe PHQ-9 scores. Those who were severely symptomatic revealed higher levels of CRP in their sera. A negative correlation between oxytocin and CRP was noticed. ROC analyses were applied to test the diagnostic ability of oxytocin and CRP for severe MDD. CRP exposed better expectedness to discriminate those with severe from mild MDD: AUC=0.731, sensitivity=0.78, specificity=0.60, and p>0.08. While oxytocin displayed poorer predictability: AUC=0.560, specificity=60, sensitivity=40 and p>0.05.
Conclusion: A significant difference in the mean levels of serum inflammatory marker (CRP) but not oxytocin between those having mild and severe PHQ-9 depressive scores. Those who were severely symptomatic revealed higher levels of CRP in their sera. A negative correlation between oxytocin and CRP was noticed.
Keywords
Major depressive disorder •Patient depression •Physiological
Introduction
Major Depressive Disorder (MDD) is a common mental illness. Universally, over 264 million persons of different ages complaining of depression. The diagnosis of MDD is entirely clinical, and at present, there is no specific diagnostic biomarker for MDD [1,2]. Depression is unlike usual temper instabilities and short-lived expressive reactions to daily life challenges. Expressly when long-term or with moderate-severe intensity, MDD may turn into a grave condition, that can cause suicide [2]. Even though MDD is not exactly an inflammatory disorder, inflammation displays a substantial input [3], which may even predict the new onset of depressive thoughts [4].
As a "nonspecific acute phase reactant" synthesized in the liver cells as an inflammatory response, C-reactive protein (CRP) is a marker of "low-grade inflammation as noted by overwhelming shreds of evidence [5-9]. Yet, whether CRP regulates or amplifies the immune response has to be fully clarified, [10-12]. Consequently, both depression and CRP are related to inflammation; nonetheless, the exact mechanism of their possible association is still unclear. Several epidemiological studies have described a relationship between depressive symptoms and higher CRP in the blood [13-15]. Some scientists assume a likely neuroimmune psychological interaction between negative affect (depressive attitude, anger, anxiety and poor prosperity), and inflammatory responses [16].
Oxytocin is a neuropeptide hormone has wide physiological effects with the ability to support health and impact behavior as revealed by the growing evidence for its actions through the immune response and as an anti-inflammatory/antioxidant [17,18]. Contrarily, the immune constituents also regulate, via its action as a binding protein, the CD38 synthesis that regulates oxytocin's ability to cross membranes [19]. The data concerning the influence of oxytocin hormone in MDD is somewhat limited. Nevertheless, there are strong pieces of evidence concerning these traits from researches that inspected oxytocin in postnatal depressive attacks, where low oxytocin concentrations were usually disposing to depression [20].
Our aim in this work was to assess the inflammatory associations of peripheral oxytocin and CRP levels in depression, among the adult age group with MDD.
Materials and Methods
Study design and sample collection
This observational study had included 180 patients, and the local authority of the health directorate had authorized its protocol. The selected patients had been recognized as MDD and were nominated by the psychiatrists at the regional main hospital (including the author) has conducted the study. Every candidate (or relatives) had completed a written conversant agreement.
Criteria for MDD patients
The diagnoses of MDD in all the included 180 adults are made after their fulfillment of "DSM-5 criteria for MDD version 7.0.2", using the "Mini International Neuropsychiatric Interview" [21]. The criteria of selection included MDD patients, attending psychiatric outpatients’ health center at Merjan hospital in Babylon province, through their follow up schedules, of both sexes, and being on antidepressants preparations for at best 4-months. A self-administered form had applied for evaluating the severity of depression according to the criteria of MDD by using a 9-items questionnaire based on "Patient Health Questionnaire depression module (PHQ-9)"[22]. MDD applicants identify the frequency of every idea arisen all over the past week "(1=not at all, 2=several days, 3=half days, and 4=nearly every day) "; where upper scores representing higher thought incidence. The total score is the calculation of queries from 1 to 30 and labels the frequency of ideas.
The patients were excluded if they had any neural illnesses such as convulsion, degenerative or traumatic brain disorders, drugs addiction, and corticosteroid consumers during the past couple of months. A total score more than or equal to ten is 88% specific and 88% sensitive for MDD [23].
The patients were divided into 5-classes; (0-4: no depression), (5- 9: mild depression), (10-14: Moderate depression), (15-20: Moderately severe) and (21-27: Severe depression). The psychologists had recognized that MDD must not be proven or excluded depending on PHQ-9 criteria only. Nevertheless, a critical diagnosis is finalized based on how well the patient recognized the analyses form, in addition to other associated information gained from the patient or relatives [22].
Biochemical assays
Samples of blood had been drained, centrifuged, and freeze for further analyses. The specimen timing was identical among all the interviewees. CRP values were estimated by a "High sensitivity immunoturbidometric assay" by immunology analyzer "Roche Diagnostics Cobas c 111 (USA)". Oxytocin hormone was assessed using Elabscience® ELISA kit. Added, the anthropometric measurements of the patients including weight, height, and body mass index had exactly calculated.
Statistical investigates
The data had grouped, arranged, processed, and scrutinized with SPSS (V-23) software. Variations in demographic variables were calculated using t-tests for continuous variables and Pearson correlations had used to judge associations between the parameters. ROC curve had used to inspect the predictive ability of both CRP and oxytocin severe depressive symptoms.
Results
Basal characteristics
Basal demographic features of the studied participants are shown in Table 1. The mean serum CRP levels were relatively high (9.0 ± 9.2 mg/ ml) among MDD patients, whereas the mean oxytocin was 33.5 pg/ml. The mean ages of the MDD patients were 39.5 ± 0.9 years and most of the patients were obese, BMI 32.9 ± 15.8 kg/m2.
| Variables | Mean ± SD |
|---|---|
| CRP (mg/ml) | 9.01 ± 9.2 |
| Oxytocin (mg/ml) | 33.5 ± 13.3 |
| Age/years | 39.5 ± 15.8 |
| BMI (kg/m2) | 32.9 ± 15.1 |
The proportion of a male to female was 1.64 to 1. In all study variables, there were non-significant changes between both sexes other than oxytocin, which was higher among females significantly (p<0.05) (Table 2).
Table 3 revealed that the correlation of the mean serum CRP and oxytocin levels with BMI, and age, which was non-significant. A negative correlation between oxytocin and CRP was noticed.
|
|
Oxytocin | CRP | BMI | Age | |
|---|---|---|---|---|---|
| CRP (mg/ml) | r | -0.02 | - | 0.139 | 0.11 |
| p | 0.85 | - | 0.19 | 0.3 | |
| Oxytocin(pg/ml) | r | - | -0.02 | 0.092 | 0.04 |
| p | - | 0.85 | 0.391 | 0.6 |
| Males (n-70) | Females (n-110) | Significance | |
|---|---|---|---|
| CRP (mg/ml) | 7.5 ± 1.4 | 10.2 ± 1.3 | >0.05 |
| Oxytocin (pg/ml) | 30.4 ± 2.3 | 60.4 ± 16.8 | 0.05 |
| Age/years | 36.9 ± 2.7 | 41.1 ± 2.1 | >0.05 |
| BMI (kg/m2) | 32.9 ± 4.4 | 32.6 ± 0.9 | >0.05 |
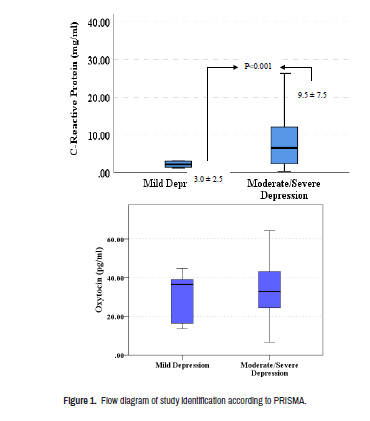
No significant deviations in the distribution of CRP and oxytocin levels according to the severity of depression scores (Table 4). As well, the mean serum levels of both CRP and oxytocin were not significantly differed among different sorts of antidepressant agents among patients with MDD (results not displayed).
| PHQ-9 Severity of Depression |
Number | CRP Mean ± SD |
P-value | Oxytocin Mean ± SD |
P-value |
|---|---|---|---|---|---|
| No depression | 0 | 0 |
>0.05 |
0 |
>0.05 |
| Mild depression | 12 | 3.0 ± 2.4 |
30.08 ± 14.1 |
||
| Moderate depression | 50 | 10.3 ± 8.7 | 33.9±12.5 | ||
| Moderate-Severe | 118 | 9.2 ± 9.7 | 33.2±13.9 | ||
| Severe | 0 | 0 | 0 |
Figure 1 revealed a significant difference in the mean levels of serum CRP between those having mild and those with severe PHQ-9 depression scores. Those who were severely symptomatic revealed higher levels of CRP in their sera (p<0.001).
No significant differences in the levels of oxytocin between those having mild and those with severe PHQ-9 scores (p>0.05), (Figure 2).
Research operation characteristics were verified for the ability of CRP and oxytocin to diagnose severe depression from those who have no or mild depressive feelings. It revealed poor predictability of oxytocin to distinguish: AUC=0.560, sensitivity=0.60, specificity=0.40 and p-value>0.05. While CRP revealed significant diagnostic ability to distinguish severe depression: AUC=0.731, specificity=0.60, sensitivity=0.78 and p-value=0.08 (Table 5).
| Variables | AUC | Specificity | Sensitivity | P-value | 95% CI | |
|---|---|---|---|---|---|---|
| Oxytocin | 0.560 | 0.60 | 0.40 | 0.65 | 0.290 | 0.829 |
| CRP | 0.731 | 0.60 | 0.78 | 0.08 | 0.576 | 0.885 |
Discussion
To the best of our knowledge, this is the first study in Iraq that measure associations among the incidence of depressive cognitions and either CRP or oxytocin levels, in individuals with MDD among Iraqi adults. The main findings of our study were higher CRP levels among MDD patients, a negative correlation between CRP and oxytocin, and a lack of association of both CRP and oxytocin with PHQ-9 scores.
The study exposed a direct association between depressive perceptions and inflammation, where the mean CRP levels were significantly greater in MDD patients with moderate to severe depression compared to those with mild depression (Figure 1). Supporting our results, a growing body of evidence in the existing eras has exposed a close relationship between inflammation, cytokines levels, and MDD. The role of the immune system in psychiatric diseases and mental comfort is a model of the commonest in this background [3,4,24]. The "macrophage model of depression" adopts the release of the pro-inflammatory cytokine from the macrophages upon activation, foothold the onset or aggravation of MDD [25]. Additionally, in a modern large meta-analysis, wide-ranging disparities in inflammatory responses have been observed in depressed patients like greater values of "TNFα, IL-6, IL-13, IL-18, IL-12, IL-1RA, and sTNFR2", along with a fall in the cytokine IFNγ [26].
Disobediently, a restricted number of investigators are revealing varying outcomes of immune activation or suppression in MDD. Both may supervene in the same subject, such as suppressed activity NK and Tregcell together with monocytes activation [27]. What's more, the scholars found that the link among MDD, immune responses and covariates are doubtless greatly multivariable and multidimensional that warrant further exploration [28]. Recently, a review has defined a bidirectional link between MDD and inflammation [29]. The polygonal relationship covers the chance of reverse causality; where depression is not a result, instead the reason of greater inflammatory levels [28].
Horn et al, it had discussed the "replication and reproducibility issues" in the association between CRP and MDD. He found a minor correlation (r-0.05), after adjustment of confounders including patients’ age, gender, weight, comorbidities, and drugs or sociopsychological issues. He also found that the influential size was tremendously attenuated and even become not significant in the analysis of high-quality scoring [30].
The role of gender on CRP levels may be more intricate in the perspective of depressive thoughts. Several researches principally studying CRP and MDD have reported inconsistent results, where some described elevated CRP values in males but not females [31], while other surveys publicized-similar to our findings, that the females had higher oxytocin serum levels [32].
Another confounder is obesity, in which data puts forward obesity as a determinant link between CRP and BMI [33]. Of note, obesity in our studied population was common.
The oxytocinergic biological system is indulged in an array of intricate social activities and contributes to emotional, social handling and behavior [34]. Even though the pieces of evidence are not decisive at current, further studies are justified to conclude the precise role of oxytocin in MDD, and whether it could be of therapeutic assistance in MDD patients [35]. The level of oxytocin, or "happy hormone", is decreased in many psychiatric illnesses, including depression [35,36].
It is salient to stress that there is no distinct normal oxytocin level [37]; it is thus hard to critic whether the measures in this patient group were high or low. The levels of blood also differ concerning illness, ages, and gender; hence, it is crucial to inspect the oxytocin levels in illness excluding those that have been formerly described, and to include a large population sample.
In this study, and similar to other studies the oxytocin levels were negatively associated with CRP and did not predict the severity of depressive symptoms. The data gathered in this study suggests that oxytocin was not strongly associated with depressive symptomatology. This finding is consistent with several other studies [37-40]. Van Londen et al. in their case-control research had found no alteration in mean oxytocin values between the two groups but did notice more disparity within the MDD patients [40]. In the same way, Cyranowski et al. stated greater unevenness in pulsatile oxytocin levels during two intervals (of one hour) in MDD females compared with control [41]. This might put forward a changed circadian oxytocin secretion in MDD, however, no follow-up revisions have inspected this hypothesis [35]. A negative association between oxytocin and severity of depressive thoughts in MDD has also been reported by others [42].
In the current study, we found that serum oxytocin levels had a negative correlation with the CRP in patients with MDD. The bulk of evidence reveals the likelihood that oxytocin could have anti-inflammatory properties and control the immune responses by reducing the release of pro-inflammatory cytokines like IL-1b, IL-6, TNF-α, NO, and glutamate [43]. Oxytocin has anti-inflammatory activity via abating the macrophages’ transition into a pro inflammatory type causing inhibited NF-κB signaling a transcription factor for a pro inflammatory immune response [44].
Consequently, it looks that serum oxytocin might be associated with believes’ spectrum in depression. Moreover, and mainly in females, serum oxytocin levels may be-less or high varied in MDD compared to the control group, still larger works evaluating serum oxytocin all over the day are essential to evaluate this [35].
The prospective for these synergistic compound interfaces of oxytocin with other neurotransmitters and neuropeptides emphasize its impact in the fine-tuning of emotion, stress, and sociability, which outline behavior and mental wellbeing. Nevertheless, the recent researches are not decisive about the role of oxytocin in MDD. We are merely starting to apprehend the in-depth molecular mechanisms of oxytocin actions at the neural level. Likewise, valuation of the role of oxytocin in further Indo-phenotyping depression is justified, to inspect cognitive dysfunction and sleep disorders. Supplementary works should highlight the exact mechanisms elaborated in the association of inflammation, oxytocin, and MDD.
The study has some limitations which had to be considered. The small population size might have subsidized the lack of significance of the outcomes. Depressive signs and negative thoughts were evaluated over the past week; in line with a valuation of state instead of long-term behaviors.
Conclusion
Taken together, the existing study among patients with MDD revealed that a significant difference in the mean levels of serum inflammatory marker (CRP), but not peripheral oxytocin, between those having low and those having higher PHQ-9 scores. Those who were severely symptomatic revealed higher levels of CRP in their sera. A negative correlation between peripheral oxytocin and CRP was noticed.
References
- Erdenen F, CS, Karşidağ and Altunoğlu E. “Evaluation of Disability, Anxiety and Depression in Hemodialysis Patients.” Nobel Med 6 (2010): 39-44.
- Abbas, Amjed H, Muna Abdulridha Rasheed, Hayder Abdul-Amir Al-Hindy, Mazin J Mousa and Hadeel Abd Ameir Al-Shalah. “The Role of Serum IL-1β in Combination with Fractional Exhaled Nitric Oxide in the Diagnosis of Adult Bronchial Asthma.” Neuro Quantology 19 (2021): 13.
- Chieh-Hsin Lee and F Giuliani, “The Role of Inflammation in Depression and Fatigue.” Front Immunol 10 (2019) 1696-1696.
- Ernst, Mareike, Elmar Brähler, Daniëlle Otten and Antonia M Werner, et al. “Inflammation Predicts New Onset of Depression in Men, but not in Women within a Prospective, Representative Community Cohort.” Sci Rep 11 (2021): 1-12.
- Hayder, Maki, Mazin Jaafar, Thekra A Alkashwan and Ahmed Sudan, et al. “On Admission Levels of High Sensitive C-Reactive Protein as A Biomarker in Acute Myocardial Infarction: A Case-Control Study.”India J Public Health Res Development 10 (2019): 5.
- Qasim Jawad AL-Daami, HSB Ghada H Naji and Hayder AA Makki. “High Sensitivity C-reactive Protein Assessment in Bronchial Asthma: Impact of Exhaled Nitric Oxide and Body Mass Index.” Sys Rev Pharm 11 (2020): 705-711.
- Al-Mumin, Amir, Hayder Abdul-Amir Makki Al-Hindy and Mazin Jaafar Mousa. “Combined Assessments of Multi-panel Biomarkers for Diagnostic Performance in Coronary Artery Disease: Case-Control Analysis.” Sys Rev Pharmacy 11 (2020): 665-671.
- Shaker, Asseel K, Raghdan Al-Saad, Raad Jasim and Hayder Abdul-Amir Makki Al-Hindy. “Biochemical Significance of Cystatin-C and High-Sensitive CRP in Patients with Acute Coronary Syndrome; any Clinical Correlation with Diagnosis and Ejection Fraction.” Sys Rev Pharmacy 11 (2020): 301-308.
- Al-Hindy, Hayder Abdul-Amir Makki, Ali Jihad Hemid Al-Athari and Mazin J Mousa, et al. “The Utility of Serum IL-1 [beta] and CRP Together with Fractional Exhaled Nitric Oxide in the Diagnosis of Asthma in Adults.” Neuro Quantology 19 (2021): 119-124.
- Abdul_Husseein, Hajir Karim, Fouad Sharif Dleikh and Ameera Jasim Al-Aaraji, et al. “Biochemical Causal-Effect of Circulatory Uric Acid, and HSCRP and their Diagnostic Correlation in Admitted Patients with Ischemic Heart Diseases.” J Cardiovascular Dis Res 11(2020): 25-31.
- Raghdan Z, AKS, Dleikh F, Al-Hindy H. “Is There any Association Between Highly Sensitive C-Reactive Protein and Dental-Status in Ischemic Heart Diseases? A Comparative Study.” Biochemical Cellular Arch 20 (2020): 6069-6075.
- Mohammed, Samer Majeed, Aliaa Sameer Hasan, Hayder Abdul Amir Makki Al-Hindy and Mazin J Mousa. “C-Reactive Protein is Associated with the Severity of Periodontal Disease? An Observational Study Among Acute Myocardial Infarction Patients.” Sys Rev Pharmacy 11 (2020): 252-257.
- Danner, Marion, Stanislav V Kasl, Jerome L Abramson and Viola Vaccarino. “Association between Depression and Elevated C-Reactive Protein.” Psychosom Med 65 (2003): 347-356.
- Fond, Guillaume, JA Micoulaud-Franchi, M Faugere and L Boyer, et al. “Abnormal C-reactive Protein Blood Levels as a Specific Biomarker of Major Depression and Non-Remission Under Antidepressants in Schizophrenia.” Prog Neuropsychopharmacol Biol Psychiatry 97 (2020): 109800.
- Maria C Pitharouli, Kylie P Glanville, Jonathan RI Coleman and Matthew Hotopf, et al. “Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank.” Am Psychiatry 178 (2021): 522-529.
- Ma, Yunsheng, David E Chiriboga, Sherry L Pagoto and Milagros C Rosal, et al. “Association Between Depression and C-reactive Protein.” Cardiol Res Pract 22(2011): 1-9.
- Bordt, Evan A, Caroline J Smith, Tyler G Demarest and Staci D Bilbo, et al. “Mitochondria, Oxytocin, and Vasopressin: Unfolding the Inflammatory Protein Response.” Neurotox Res 36 (2019): 239-256.
- Abdulamir, Haidar A, Omar F Abdul-Rasheed and Emad A Abdulghani. “Low Oxytocin and Melatonin Levels and their Possible Role in the Diagnosis and Prognosis in Iraqi Autistic Children.” Saudi Med J 37 (2016): 29.
- Yasuhiko, Yamamoto and Higashida Haruhiro. “RAGE Regulates Oxytocin Transport into the Brain.” Comm Biol 3 (2020): 1.
- Massey, Suena H, Stephanie A Schuette, Hossein Pournajafi-Nazarloo and Katherine L Wisner, et al. “Interaction of Oxytocin Level and Past Depression May Predict Postpartum Depressive Symptom Severity.” Arch Womens Ment Health 19 (2016): 799-808.
- Sheehan, David V, Yves Lecrubier, K Harnett Sheehan and Patricia Amorim, et al. “The Mini-International Neuropsychiatric Interview (MINI): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10.” J Clin Psychiatry 59 (1998): 22-33.
- Kroenke, Kurt, Robert L Spitzer and Janet BW Williams. “The PHQ‐9: Validity of a Brief Depression Severity Measure.” J Gen Intern Med 16 (2001): 606-613.
- NIMH. “Depression Basics”. 5 (2016): 1.
- Osimo, Emanuele Felice, Luke James Baxter, Glyn Lewis and Peter B Jones, et al. “Prevalence of Low-Grade Inflammation in Depression: A Systematic Review and Meta-Analysis of CRP levels.” Psychological Med 49 (2019): 1958-1970.
- Smith, Ronald S. “The Macrophage Theory of Depression.” Med Hypotheses 35 (1991): 298-306.
- Köhler, Cristiano A, Thiago H Freitas, M de Maes and NQ De Andrade, et al. “Peripheral Cytokine and Chemokine Alterations in Depression: A Meta‐Analysis of 82 Studies.” Acta Psychiatr Scand 135 (2017): 373-387.
- Grosse, Laura, Thomas Hoogenboezem, Oliver Ambrée and Silja Bellingrath, et al. “Deficiencies of the T and Natural Killer Cell System in Major Depressive Disorder: T Regulatory Cell Defects are Associated with Inflammatory Monocyte Activation.” Brain 54 (2016): 38-44.
- Fried, Eiko I, S Von Stockert, J MB Haslbeck and F Lamers, et al. “Using Network Analysis to Examine Links between Individual Depressive Symptoms, Inflammatory Markers, and Covariates.” Psychol Med 50 (2020): 2682-2690.
- Beurel, Eléonore, Marisa Toups, and Charles B. Nemeroff. “The Bidirectional Relationship of Depression and Inflammation: Double Trouble.” Neuron 107 (2020): 234-256.
- Horn, Sarah R, Madison M Long, Benjamin W Nelson and Nicholas B Allen, et al. “Replication and Reproducibility Issues in the Relationship between C-Reactive Protein and Depression: A Systematic Review and Focused Meta-Analysis.” Brain Behav Immun 73 (2018): 85-114.
- Haefner, Sibylle, Rebecca T Emeny, Maria Elena Lacruz and Jens Baumert, et al. “Association between Social Isolation and Inflammatory Markers in Depressed and Non-depressed Individuals: Results from the MONICA/KORA Study.” Brain Behav Immun 25 (2011): 1701-1707.
- Nazmi, Aydin, IO Oliveira and Cesar G Victora. “Correlates of C-Reactive Protein Levels in Young Adults: A Population-Based Cohort Study of 3827 Subjects in Brazil.” Braz J Med Biol Res 41 (2008): 357-367.
- Timpson, Nicholas J, Børge G Nordestgaard, Roger M Harbord and Jeppe Zacho, et al. “C-reactive Protein Levels and Body Mass Index: Elucidating Direction of Causation Through Reciprocal Mendelian Randomization.” Int J Obes (Lond) 35 (2011): 300-308.
- Ross, Heather E and Larry J Young. “Oxytocin and the Neural Mechanisms Regulating Social Cognition and Affiliative Behavior.” Front Neuroendocrinol 30 (2009): 534-547.
- Slattery, David A and Inga D Neumann. “Oxytocin and Major Depressive Disorder: Experimental and Clinical Evidence for Links to Aetiology and Possible Treatment.” Pharmaceuticals 3 (2010): 702-724.
- Frasch, A. “Reduction of Plasma Oxytocin Levels in Patients Suffering from Major Depression.” Adv Exp Med Biol 395 (1995): 257-258.
- Miwa, Yusuke, Hidekazu Furuya, Ryo Yanai and Tsuyoshi Kasama, et al. “The Relationship between the Serum Oxytocin Levels, Disease Activity, the ADLs and the QOL in Patients with Rheumatoid Arthritis.” Intern Med (2017): 9191-17.
- Nishi, Daisuke, Kenji Hashimoto, Hiroko Noguchi and Yoshiharu Kim, et al. “Serum Oxytocin, Posttraumatic Coping and C-Reactive Protein in Motor Vehicle Accident Survivors by Gender.” Neuropsychobiology 71 (2015): 196-201.
- Yukselmis, Ozkan. “Serum Oxytocin Levels in Patients with Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthritis and their Association with Disease Activity.” Aktuelle Rheumatologie (2021): 46 400-405.
- Van Londen, Liesbeth, Jaap G Goekoop, Godfried MJ Van Kempen and Ank C Frankhuijzen-Sierevogel, et al. “Plasma Levels of Arginine Vasopressin Elevated in Patients with Major Depression.” Neuropsychopharmacology 17 (1997): 284-292.
- Cyranowski, Jill M, Tara L Hofkens, Ellen Frank and Howard Seltman, et al. “Evidence of Dysregulated Peripheral Oxytocin Release Among Depressed Women.” Psychosom Med 70 (2008): 967.
- Scantamburlo, Gabrielle, Michel Hansenne, Sonia Fuchs and William Pitchot, et al. “Plasma Oxytocin Levels and Anxiety in Patients with Major Depression.” Psychoneuroendocrinology 32 (2007): 407-410.
- Yuan, Lin, Song Liu, Xuemei Bai and Yan Gao, et al. “Oxytocin Inhibits Lipopolysaccharide-Induced Inflammation in Microglial Cells and Attenuates Microglial Activation in Lipopolysaccharide-Treated Mice.” J Neuroinflammation 13 (2016): 1-17.
- Tang, Yan, Yao Shi, Yifei Gao and Xiaomeng Xu, et al. “Oxytocin System Alleviates Intestinal Inflammation by Regulating Macrophages Polarization in Experimental Colitis.” Clin Sci 133 (2019): 1977-1992.
Citation: Alhaideri, Amer Fadhil, Azher Nema Mohammed Al- Agam, Hayder Abdul-Amir M Al-Hindy and Mazin J Mousa, et al. “Inflammatory Associations of Peripheral Oxytocin, C-Reactive Protein Levels with Depression Among Adult Age Group with Major Depressive Disorder.” Clin Schizophr Relat Psychoses 15S (2021) Doi: 10.3371/CSRP.AAAM.221021.
Copyright: © 2021 Alhaideri AF et al, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.







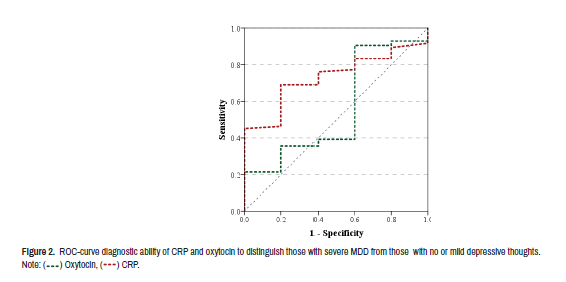
 ) Oxytocin, (
) Oxytocin, (  ) CRP.
) CRP.