Brief Report - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Impact Of Apurinicapyrimidinic Endonuclease Asp148Glu Rs3136820 Gene Polymorphism And Ape1 Level In Depression Disorders
Mona N. Al-Terehi1, Zahraa F. AL-Khero2, Abeer Ameen Baqer3, Mohammed Abed Jawad4*, Safa K. Hachim5,6, Samah Sajad Kadim7, Ali Ahmed Nayyef8 and Sarah Hussein Ali Alallo92Department of Medical Laboratory Techniques, University of Mashreq, Mashreq, Iraq
3Department of Medical Laboratory Techniques, Dijlah University, Baghdad, Iraq
4Department of Medical Laboratory Techniques, Al‐Nisour University, Baghdad, Iraq
5Department of Medical Laboratory Techniques, The Islamic University, Najaf, Iraq
6Department of Pharmacy, Osol Aldeen University, Baghdad, Iraq
7Department of Medical Laboratory Techniques, AL‐Mustaqbal University, Babil, Iraq
8Department of Pharmacy, Al‐Nisour University, Baghdad, Iraq
9Department of Dentistry, Al‐Zahrawi University, Karbala, Iraq
Mohammed Abed Jawad, Department of Medical Laboratory Techniques, Al‐Nisour University, Baghdad, Iraq, Email: mohammed.a.medical.lab@nuc.edu.iq
Received: 05-Nov-2021 Accepted Date: Nov 19, 2021 ; Published: 26-Nov-2021, DOI: 10.3371/.CSRP.AMZA.112621
Abstract
The repair system processes are very important in the maintenance of genome against mutagenic factors, the present study deal with Apurinic/apyrimidinic endonuclease gene polymorphism and its level in depression disorder patients, blood samples were collected from patients and control to serum separation for APE1 detection and DNA extraction for APE1 Asp148Glu (rs3136820) gene polymorphism by CTTP-PCR technique, finding of present research show that the mean age of patients and control were non-significant differences (P 0.156), the BMI was significant differences (P 0.042) between groups. non- significant decrement was appeared in the APE1 level in the patients group (P 0.183), the CTTP-PCR products show two alleles (T and G) and three genotyping in addition to deletion mutation (TT, GT and GG), significant differences was observed in TG allele that low frequent in patients than the control group (OR 5.0909, CI% 1.3190-19.649, P 0.0182). The GG allele shows high frequent in patients than the control group in non-significant differences (OR 3.8500, CI 95% 0.7614-19.468, P 0.103). Deletion mutation also found in non-significant differences (OR 1.7143 CI% 0.1305-22.5139, P 0.6816). The effect of APE1 genotyping in APE1 level show non-significant differences in APE1 levels, according to APE1 genotyping (P 0.870) although of decreasing the level of APE1 in TG in patients than other genotyping. There was a weak relation of APE1 levels, and no association between APE1 Asp148Glu (rs3136820) genotyping with depression disorder.
Keywords
Apurinic/apyrimidinic endonuclease • Asp148Glu (rs3136820) • Gene polymorphism • APE1 level • Depression disorders patients • CTTP-PCR
Introduction
The Apurinic/apyrimidinic endonuclease gene encodes the major AP endonuclease in human. It's used in DNA repair in DNA damage lesions by the Base Excision Repair (BER) pathway in cell nucleus and mitochondria [1], the AP Endonuclease 1 (APE1) is enzyme having more than one functions, it’s a member of the BER pathway, in addition to DNA repair activity, it has a role in the reductive many transcription factors activations [2], there are regions encoded the tow function of APE1 the redox function encoded by N-terminal region and the repair function encoded by C-terminal [3,4].
The psychiatric diseases have been found to associate with accumulated DNA damage and impaired in DNA rapier, these damages are caused by oxidative stress and the brain neurons found to be more vulnerable to oxidative destruction than other cells, resulted to pronounced neuropathology, like mutations, dysfunction in some cells and aberrant phenotypes [5-10].
Depression disorders have been recorded in high incidence in Iraq after COVID-19 era which leads to lifestyle alteration in population, thus the present study was suggested to evaluate the Apurinic/apyrimidinic endonuclease gene polymorphism in APE1 level in depression disorder patients.
Materials and Methods
Sample collection and study sitting: the present study, including 20 cases (male) have age range (20 years to 66 years) years and 25 healthy contributors have age range (19 years to 58 years), patients suffered from depression disorder symptoms who attended to the privet Psychiatric Clinic and diagnosis by A specialist doctor, blood samples were collected according to ethical approval of the ministry of environment and health of Iraq, blood samples divided into two parts, for sera isolation and DNA extraction, both parts stored -20ºC until its used.
APE1 level detection
The APE1 detected by ELIZA by E6642Hu kit provided from bioassay technology Lab with high sensitivity (0.099 ng/ml).
DNA extraction
About 200 μl of blood was used to DNA extraction with proteinase K according to manufacture leaflet (favorgen), the concentration and purity were detected using Nano drop.
Oligos and PCR experiment
The APE1 Asp148Glu (rs3136820) polymorphism using the following primers that provided from macrogene company 5′-CCT ACG GCA TAG GTG AGA CC; R1:5′-TCC TGA TCA TGC TCC TCC-3’; F2: 5′-TCT GTT TCA TTT CTA TAG GCG AT; R2: 5′-GTC AAT TTC TTC ATG TGC CA [11]. The final concentration of Oligos aliquot was 10 p/μl, the CTTP-PCR was used annealing Tm 58ºC to amplification target loci, the products were Three bands a 236 bp, 167 bp and a 360 bp band for T allele, G allele and common band respectively.
Gel electrophoresis
Agaros was used to visualize the extraction DNA and the amplification products using 70 V, 20 mA, 1% agaros, 0.5X TBE buffer for 45 min and ethidum bromide staining.
Results
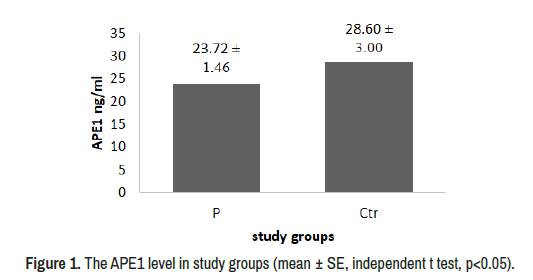
The results of present research show that the mean age of patients and control were (39.500 ± 3.104), (33.88 ± 2.436) in non-significant differences (P 0.156), the BMI was (25.01 ± 0.864) for patients and (27.41 ± 0.759) for control in significant differences (P 0.042). Also non- significant decrement was appeared in APE1 level in patients group (P 0.183) (Figure 1).
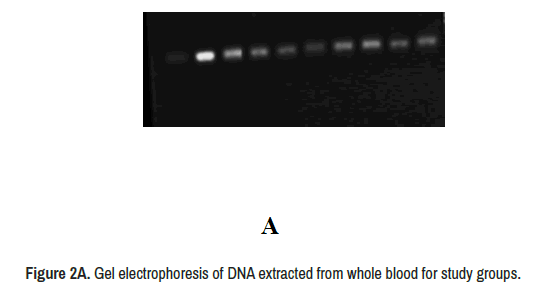
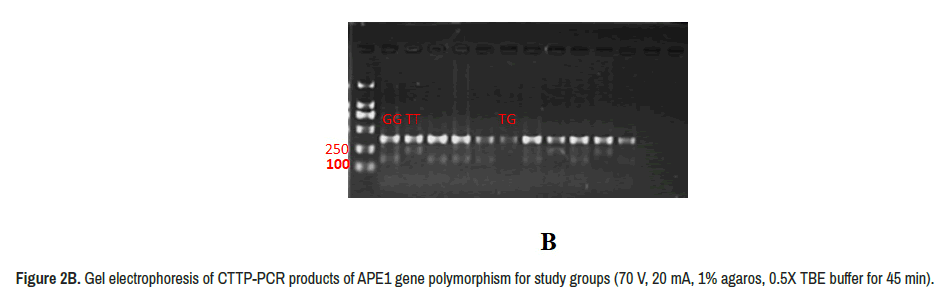
The DNA extracted from whole blood show concentration ranged (65 ng/μl to 130 ng/μl) and purity ranged (1.6-2.3), on band was observed for each sample (Figure 2A). The PCR products show two alleles (T and G) and three genotyping in addition to deletion mutation (TT, GT and GG) (Figure 2B).
The statistical analysis of genotyping in patients and control groups is clarified in Table 1, significant differences was observed in TG allele that low frequent in patients (20%) than control (56%) (OR 5.0909, CI% 1.3190-19.649, P 0.0182). The GG allele shows high frequent in patients (35%) than control (15%) in non-significant differences (OR 3.8500, CI 95% 0.7614-19.468, P 0.103). Deletion mutation also found in (20%) and (4%) in control group in non-significant differences (OR 1.7143 CI% 0.1305- 22.5139, P 0.6816), the allele frequency show that G more frequent in patients than T allels (Table 1).
| Genotyping | Patients (%) | Control (%) | Odd ratio (CI%) | Sig |
|---|---|---|---|---|
| TG | 4(20%) | 14(56%) | 5.0909 (1.3190-19.649) |
0.0182 |
| TT | 5(25%) | 7(28%) | ||
| GG | 7(35%) | 3(15%) | 3.8500 (0.7614-19.468) |
0.1030 |
| G | 0.562 | 0.416 | ||
| T | 0.437 | 0.583 | ||
| Deletion mutation | 4(20%) | 1(4%) | 1.7143 (0.1305 to 22.5139) |
0.6816 |
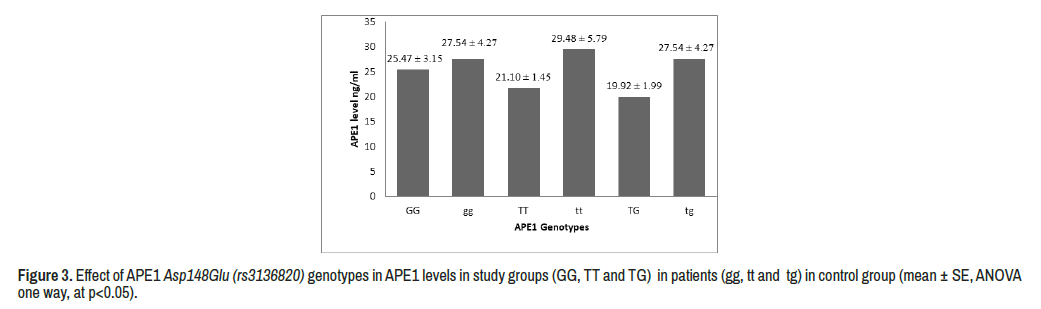
The effect of APE1 genotyping in APE1 level was detected for study subjects, there was non-significant differences in APE1 level according to APE1 genotyping (P 0.870) although of decreasing the level of APE1 in TG in patients than other genotyping ( Figure 3).
Discussion
The present suggestion was conducted to estimate the an important repair enzyme with its encoded gene in subjects with depression regarding to prolonged psychological stress lead to increased oxidative stress which may be caused DNA mutation, present finding shows non-significant decreased in APE1 level in patients, the depletion of APE1 was found to be associated with neuronal death after ischemic brain injury [12]. Stetler, et al. indicated that the DNA repair enzymes like APE1 stimulation was a unique strategy for neuroprotection against hippocampal injury [13]. Take together APE1 is a critical cellular protein has multifunction like a transcriptional cofactor, and a suppressor of ROS by a redox site [14], these functions could lead to neuro-protection independent of DNA repair. Investigations clarified that the deficiency in the expression and activity of APE1 exacerbates oxidative injury in multiple models, including neurons [15-17]. The depletion in APE1 may be contributed in depression due to its role in the neuron cells protection against high level of oxidative stress biomarkers that have been proved in patients group (data not shown). The APE1 Asp148Glu (rs3136820) genotyping show non-significant association with depression patients in present finding, another study show a significant association between repair genes and schizophrenia included APE1 [18]. The polymorphism in DNA repair genes may be effected in protein levels which contributed in rapier pathway have been associated with different disease like neurological disorders [19] like Alzheimer's disease [20], Parkinson's disease [21] and Huntington's disease [22], the impact of (rs3136820) in the APE1 level show that tg, tt and d genotyping have a higher level than others and (TT and TG) genotyping were low frequent in patients than the control group, thus the lower level of APE1 in patients may be because more frequent of these genotyping in the patients group. Other DNA repair enzyme genes also studied in Iraqi Depression patients; the results show no association between RAD-18 and XRCC1 genotyping [23]. The relation between APE1 gene polymorphism with depression didn’t observe in the previous studies [24,25].
Conclusion
The association of DNA repair with some disease should be validated; the present study needs more investigations to prove other APE1 SNPs relation relations to depression disorder especially suffered from unbalanced in oxidative stress. However, it can be concluded that the APE1 level and APE1 Asp148Glu (rs3136820) genotyping may have an effect in the depression disorders and contributed in its pathology.
References
- D’Errico, Mariarosaria, Eleonora Parlanti and Eugenia Dogliotti. "Mechanism of Oxidative DNA Damage Repair and Relevance to Human Pathology." Mutat Res 659 (2008): 4-14.
- Bhakat, Kishor K., Anil K. Mantha and Sankar Mitra. "Transcriptional Regulatory Functions of Mammalian AP-Endonuclease (APE1/Ref-1), an Essential Multifunctional Protein." Antioxid Redox Signal 11 (2009): 621-37.
- Xanthoudakis, Steven, Graham G. Miao and Tom Curran. "The Redox and DNA-Repair Activities of Ref-1 are Encoded by Nonoverlapping Domains." Proc Natl Acad Sci USA 91 (1994): 23-27.
- Barzilay, Gil and Ian D. Hickson. "Structure and Function of Apurinic/Apyrimidinic Endonucleases." Bioessays 17 (1995): 713-9.
- Subba Rao, Kalluri. "Mechanisms of Disease: DNA Repair Defects and Neurological Disease." Nat Clin Pract Neurol 3 (2007): 162-72.
- Raza, Muhammad Ummear, Turan Tufan, Yan Wang and Christopher Hill, et al. "DNA Damage in Major Psychiatric Diseases." Neurotoxicity Res 30 (2016): 251-67.
- Al-Omari, Raghda SM and Mahdi H. Al-Ammar. "Association between TNF-α (-308G→ A) GenePolymorphism and Burn Patient with Sepsis." IJDDT 11 (2021): 217-21.
- Alsadawi, Aqeel A., Al-Karrar Kais Abdul Jaleel duabel and Haider A. Alnaji. "Hepatitis C and IL-6 with 174G/C GenePolymorphism in β-Thalassemia." IJDDT 9 (2019): 617-22.
- Kirtipal, Nikhil, Hitender Thakur and Ranbir Chander Sobti. "Insertion/Deletion Polymorphism of Angiotensin-Converting Enzyme and Chronic Obstructive Pulmonary Disease: A Case-Control Study on North Indian Population." Mol Biol Res Commun 8 (2019): 167-70.
- Kadhem, Esraa J. and M. Darweesh. "Association of IL-101082G/A Gene Polymorphism with its Serum Levels in Asthma Patients." Biochem Cell Arch 17 (2017): 709-13.
- Das, Sambuddha, Sukanya Purkayastha, Hirakjyoti Roy and Anima Sinha, et al. "Polymorphisms in DNA Repair Genes Increase the Risk for Type 2 Diabetes Mellitus and Hypertension." Biomolecular Concepts 9 (2018): 80-93.
- Singh, Shilpee and Ella W. Englander. "Nuclear Depletion of Apurinic/Apyrimidinic Endonuclease 1 (Ape1/Ref-1) is an Indicator of Energy Disruption in Neurons." Free Radic Biol Med 53 (2012): 1782-90.
- Stetler, R. Anne, Yanqin Gao, R. Suzanne Zukin and Peter S. Vosler, et al. "Apurinic/Apyrimidinic Endonuclease APE1 is Required for PACAP-Induced Neuroprotection against Global Cerebral Ischemia." Proc Natl Acad Sci USA 107 (2010): 3204-9.
- Tell, Gianluca, Giuseppe Damante, David Caldwell and Mark R. Kelley. "The Intracellular Localization of APE1/Ref-1: More than a Passive Phenomenon?" Antioxid Redox Signal 7 (2005): 367-84.
- Ono, Yasuhiro, Tomohisa Furuta, Takashi Ohmoto and Kosuke Akiyama, et al. "Stable Expression in Rat Glioma Cells of Sense and Antisense Nucleic Acids to a Human Multifunctional DNA Repair Enzyme, APEX Nuclease." Mutat Res 315 (1994): 55-63.
- Vasko, Michael R., Chunlu Guo and Mark R. Kelley. "The Multifunctional DNA Repair/Redox Enzyme Ape1/Ref-1 Promotes Survival of Neurons after Oxidative Stress." DNA Repair 4 (2005): 367-79.
- McNeill, Daniel R. and David M. Wilson. "A Dominant-Negative form of the Major Human Abasic Endonuclease Enhances Cellular Sensitivity to Laboratory and Clinical DNA-Damaging Agents." Mol Cancer Res 5 (2007): 61-70.
- Odemis, Sibel, Erdem Tuzun, Huseyin Gulec and Umit B. Semiz, et al. "Association between Polymorphisms of DNA Repair Genes and Risk of Schizophrenia." Genet Test Mol Biomark 20 (2016): 11-17.
- Mantha, Anil K., Bibekananda Sarkar and Gianluca Tell. "A Short Review on the Implications of Base Excision Repair Pathway for Neurons: Relevance to Neurodegenerative Diseases." Mitochondrion 16 (2014): 38-49.
- Huang, En, Dianbo Qu, Yi Zhang and Katerina Venderova, et al. "The Role of Cdk5-Mediated Apurinic/Apyrimidinic Endonuclease 1 Phosphorylation in Neuronal Death." Nat Cell Biol 12 (2010): 563-71.
- Silber, John R., Michael S. Bobola, A. Blank and Kathryn D. Schoeler, et al. "The Apurinic/Apyrimidinic Endonuclease Activity of Ape1/Ref-1 Contributes to Human Glioma Cell Resistance to Alkylating Agents and is Elevated by Oxidative Stress." Clin Cancer Res 8 (2002): 3008-18.
- Lewandowski, Nicole M., Yvette Bordelon, Adam M. Brickman and Sergio Angulo, et al. "Regional Vulnerability in Huntington's Disease: fMRI-Guided Molecular Analysis in Patients and a Mouse Model of Disease." Neurobiol Dis 52 (2013): 84-93.
- Al-Terehi, Mona N., Zahraa Haleem AlQaim and Arafat Hussein Aldujaili. "Impact of DNA Repair System Genes RAD-18 and XRCC1 Polymorphism in Depression Disorders Patients." Clin Schizophr Relat Psychoses 15 (2021): 1-4.
- Al Mashhadani, Zuhair I., Muneam H. Ali, and Mona N. Al-Terehi. "Correlation between the Dopamine and ROS Levels in Depression Disorders." IJPAQ 12(2021): 328-30.
- Alkadir, Ola K. A., Zuhair I. Al Mashhadani, Mona N. Al-Terehi and Hadeel A. Al-Rrubaei, et al. "The Estimation of Oxidative Stress from Alcohol Use Disorders in Iraqi Population." IJPAQ 12(2021): 300-02.
Citation: Al‐Terehi, Mona N., Zahraa F. AL‐Khero, Abeer Ameen Baqer and Mohammed Abed Jawad, et al. “Impact of Apurinic/ Apyrimidinic Endonuclease Asp148Glu (rs3136820) Gene Polymorphism and APE1 Level in Depression Disorders Patients.” Clin Schizophr Relat Psychoses 15S (2021). DOI: 10.3371/.CSRP.AMZA.112621.
Copyright: © 2021 Al-Terehi MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.