Review Article - Clinical Schizophrenia & Related Psychoses ( 2022) Volume 16, Issue 2
Herbs Derived Bioactive Compounds and their Potential for the Treatment of Neurological Disorders
Anubhav Dubey1,2, Niladry Sekhar Ghosh1*, Nidhee Agnihotri3, Amit Kumar4, Mayankesh Pandey5 and Shiwangi Nishad62Adarsh Vijendra Institute of Pharmaceutical Sciences, Shobhit University Gangoh, Uttar Pradesh, India
3Department of Pharmaceutical Chemistry, Maharana Pratap College of Pharmaceutical Sciences, Uttar Pradesh, India
4Department of Pharmacognosy, Advance Institute of Biotech and Paramedical Sciences, Uttar Pradesh, India
5Department of Pharmacy, Vidya Bhavan College of Pharmacy, Uttar Pradesh, India
6Department of Pharmaceutics, Shri Ramnath Singh Institute of Pharmaceutical Science and Technology, Gwalior, India
Niladry Sekhar Ghosh, Adarsh Vijendra Institute of Pharmaceutical Sciences, Shobhit University Gangoh, Uttar Pradesh, India, Email: niladry.ghosh@shobhituniversity.ac.in
Received: 18-Jul-2022, Manuscript No. CSRP-22-69360; Editor assigned: 20-Jul-2022, Pre QC No. CSRP-22-69360 (PQ); Reviewed: 04-Aug-2022, QC No. CSRP-22-69360; Revised: 11-Aug-2022, Manuscript No. CSRP-22-69360 (R); Published: 19-Aug-2022, DOI: 10.3371/CSRP.DANG.081922
Abstract
Alzheimer's Disease (AD) and other memory-related difficulties have both been treated successfully with herbs. Dementia is a neurodegenerative disease that causes the gradual deterioration of affective and cognitive capacities over time. Many factors, including poor cerebral blood flow, poison toxicity, mitochondrial dysfunction, oxidative injury, and, in some cases, the coexistence of other diseases like Alzheimer's Disease (AD), Huntington's Disease (HD), Parkinson's Syndrome (PD), and Attention Deficit Hyperactivity Disorder (ADHD), have been linked to Dementia (ADHD). Although semi-synthetic pharmaceuticals have been shown to be effective in treating Alzheimer's disease and dementia caused by AD, many of these drugs come with unwanted side effects. Therefore, traditional medicine provides a selection of plant-derived lead compounds that may prove useful in future medical research. This research examines the use of ayurvedic plants in the treatment of neurodegenerative diseases in various parts of the world. Also, it has been found that plants can protect the brain system from the damaging effects of proinflammatory cytokines including IL-6, IL-1b, and TNF-a by increasing antioxidant activity, decreasing oxidant levels, and blocking the breakdown of acetylcholinesterase. The most important ayurveda medicinal herbs and the biochemical effects they have have been highlighted. This suggests that the above medicinal herbs and their active ingredients have therapeutic potential in treating neurodegenerative disorders, such as Alzheimer's disease. and sadness, all of which have been associated to neuroinflammation and neurotransmitter dysregulation.
Keywords
Neurodegeneration • Dementia • Huntington's disease • Ayurvedic plants • Neurotransmitter dysregulation
Introduction
Neurodegenerative diseases cause slow neuronal death, which manifests as cognitive decline and sensory dysfunction due to disorders including Alzheimer's, Parkinson's, and Multiple Sclerosis. The classic trifecta of Alzheimer's disease, including senile plaques, neurofibrillary tangles, and granulovascular degeneration, is widely accepted as the disease's underlying aetiology. Loss of memory, trouble learning new material, despair, hostility, anxiety, and impaired vision are all characteristics used for diagnosis [1].
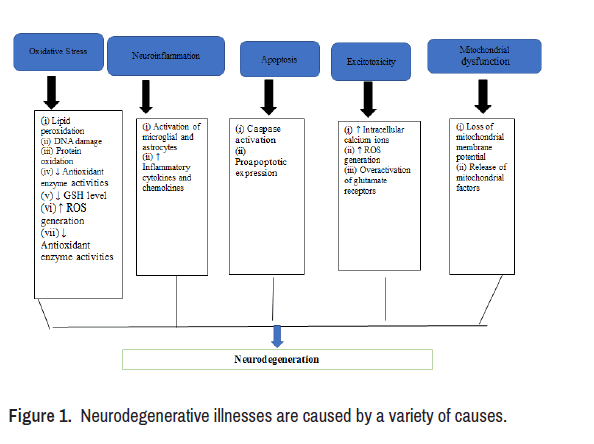
Neuroprotection refers to the ability of a mechanism to shield the Central Nervous System (CNS) from neural damage, and it applies to both acute and progressive neurodegenerative illnesses like Alzheimer's and Parkinson's. Diseases can be avoided rather than treated with herbal medication when combined with healthy lifestyle choices like eating right and getting regular exercise. Plant therapy, often known as herbal treatment, is the complementary and alternative medicine practise of making use of plant parts for therapeutic effects (leafs, branches, roots, bulbs, fruits and seeds) [2]. In light of the foregoing, it has been indicated that the Ayurvedic texts have a comprehensive assessment of the herbal substances suggested in the treatment of nervous system illnesses associated with the aforementioned ailments (Figure 1).
Neurodegenerative Treatment Targets
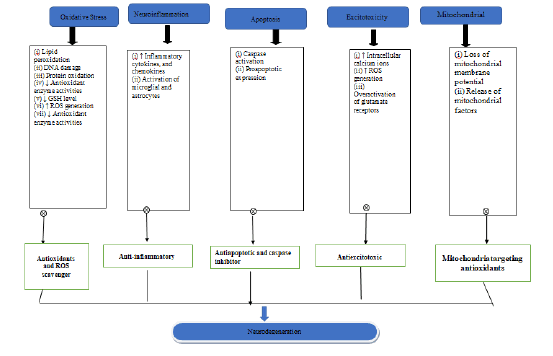
This study used ancient ayurvedic texts and databases like PubMed, Web of Science, Google Scholar, and Scopus. In vitro, animal, review papers, and clinical investigations on herbal plants with neuroprotective and behavioural alterations, oxidant/anti-oxidant characteristics, and proinflammatory cytokines were assessed (Figure 2).
Neuro Protective Herbal Plants
Ayurveda integrates philosophy, theology, and medicine into daily living. Vata, pitta, and kapha govern all cellular activities necessary for a healthy life. Vata controls activity and events, pitta health and resources, and kapha system development. When these qualities are interrupted, for as by a bad diet or unfavourable climate, diseases occur (Figure 3) [3].
Guduchi (Tinospora cordifolia)
SD causes anxiety, cognitive dysfunctions, and muscle control impairment in certain persons. In one study, 50% ethanolic Tinospora cordifolia Extract (TCE) reduced SD's harmful effects. Adult Wistar female rats were examined for cognitive ability, anxiety, and motor control in three groups: VUD, VSD, and TCE-Treated-Sleep Deprived (TSD). TSD animals performed better in fear and cognitive tests than VSD animals. TCE retherapy modulates stress-induced proliferation of plasticity markers PSANCAM, NCAM, and GAP-43 and LTP-maintaining proteins. TCE can help manage sleep deprivation-related stress and boost cognitive capacities, according to this research [4].
Ayurvedic formulas boost the body's ability to resist stress and cope with adversity. Guduchi (Tinospora cordifolia) and Madhuyashti (Glycirrhiza glabra) are Ayurvedic preparations considered to promote excellent health and healthy ageing. Using a Drosophila model, the stress-resistance effects of Guduchi and Madhuyashti were examined [5].
One study employed monosodium glutamate to injure cerebellar neurons. Four extracts were produced by fractionating an aqueous ethanolic extract of T. cordifolia: Hexane, Chloroform, Ethyl Acetate, and Butanol. Butanol Extract of T. cordifolia (B-TCE) avoided glutamate-induced neurotoxicity. Synaptic, apoptotic, inflammatory, cell cycle regulatory, and plasticity markers were examined using immunohistochemistry and Western blotting. Neurite outgrowth and migration were also studied using main explant cultures, wound scrapes, and gelatin zymograms. B-TCE administration of glutamate-treated cultures normalised downregulation of neuronal (MAP-2, GAP-43, NF200) and anti-apoptotic (B-TCE) markers (Bcl-xL). B-TCE increases cerebellar neuron proliferation, migration, and plasticity, which glutamat blocked. B-TCE administration of glutamate- treated cultures normalised downregulation of neuronal (MAP-2, GAP- 43, NF200) and anti-apoptotic (B-TCE) markers (Bcl-xL). B-TCE may have neuroprotective and neuroregenerative characteristics against glutamate-mediated toxicity, making it a prospective therapeutic target for neurodegenerative disorders [6].
Tinospora cordifolia (Tc) and Phyllanthus emblica (Pe) influenced learning and memory in mice with and without Bhavana samskara. All drugs reduced transmission latencies in mice, but had similar effects on vehicle control. After 24 hours, transfer latency decreased in all drug- treated groups. Both formulations improved learning and memory relative to Tc and Pe. Plant medicines changed learning and memory. Fixed-dose Bhavana samskara formulations demonstrated promising results, but the difference wasn't statistically significant. These medicines performed better than current medicines, thus their nootropic potential must be studied [7].
In-depth research explores Bhavana samskara using Tinospora cordifolia and Phyllanthus emblica in mice. Five groups of animals were used: sham, negative, positive, and two research groups (n=6 each). Oral gavage of 200 mg/kg and 400 mg/kg TCEE was given to experimental groups for 30 days. Researchers looked at dopamine levels, oxidative stress, complex I function, and brain iron asymmetry ratio, as well as locomotor activity including muscular coordination and catatonia. TCEE at 200 mg/kg (1.57, 0.18) and 400 mg/kg (1.11, 0.15) reduced iron asymmetry ratio. TCEE's neuroprotection was backed by reduced oxidative stress and restored locomotor function. TCEE protects dopaminergic neurons in 6OHDA-induced PD and lowers iron buildup [8].
Kapikachhu (Mucuna pruriens)
MPEP contains natural Levodopa (LD) and is free of drug-induced dyskinesias. In HP monkeys, MPEP with and without Carbidopa (CD) and LD+CD were compared. Each therapy improved Parkinsonism. Comparing the neuronal firing parameters of the SNR and STN in HP monkeys with MPEP+CD and LD+CD assessed basal ganglia circuitry changes. Both treatments decreased SNR firing rate compared to HP. LD+CD treatments increased SNR bursting fire behaviour but not MPEP+CD. STN shooting didn't change anything. In the basal ganglia, Mucuna pruriens uses new and unique LD mechanisms to improve parkinsonism without causing dyskinesias [9].
In a study, a Mucuna pruriens extract containing L-DOPA and rich new phytochemicals reduced MPTP-induced neurotoxicity through the NF-kB and pAkt pathways. The results reveal that MP extract decreased MPTP- induced neuroinflammation and reversed biochemical and behavioural deficits in PD mice, supporting its traditional use [10].
Mucuna pruriens (MP), a levodopa-containing leguminous plant that thrives in all tropical locations, was studied as an alternate source of levodopa for persons with Parkinson's Disease (PD) who can't afford long- term therapy with commercially available levodopa preparations. A single dose of MP powder produced from roasted seeds was manufactured. Eighteen patients with advanced Parkinson's disease were randomly assigned the following therapies: (1) Levodopa dispersible LD1DDCI; (2) High-dose MP (17.5 mg/kg); (3) Low-dose MP (12.5 mg/kg); (4) LD without DDCI (LD2DDCI; 17.5 mg/kg); (5) MP plus benserazide (MP1DDCI; 3.5 mg/kg); (6) Placebo. The effectiveness endpoints were on-state length and motor response improvement at 90 minutes and 180 minutes. As safety measures, adverse events, blood pressure, heart rate, and dyskinesia severity were all considered. MP-Hd exhibited 90 minutes to 180 minutes improved motor strength, longer ON time, and less dyskinesia than MP-Ld. MP-Hd produced fewer AEs than LD1DDCI and LD2DCI. No cardiovascular reactions have changed. Single-dose MP met efficacy and safety testing, unlike levodopa/benserazide. High-dose MP clinical findings had a better tolerability profile than levodopa alone [11,12].
Mucuna pruriens contains DDCI-like compounds or reduces the requirement for a second DDCI to treat parkinsonism. Parenterally administered Mucuna pruriens seed powder water extract may lead to new Parkinson's disease treatments. Dopaminergic neurotoxins (6-OHDA and rotenone) reduced intrinsic negative geotaxis activity in D. Melanogaster by 35.3% and 32.8%, respectively. MPE creates bioactive molecules that may protect against PD [13]. Flies PTEN-Induced Putative Kinase 1 (PINK1B9) mutant Drosophila Melanogaster (Dm) is used to study Parkinson's Disease physiopathology (PD). Mpe has multiple sites of action, thus its effects aren't just from L-Dopa. These findings complement clinical data that Mpe can delay chronic L-Dopa-induced motor problems. This supports using PINK1B9, Dm as a translational model to study Mucuna pruriens for Parkinson's disease [14]. Eight Parkinson's patients with short-term L-dopa response and time dyskinesias participated in a randomised, controlled, double-blind crossover experiment. Clinical effects and pharmacokinetics of L-dopa after two doses of Mucuna were compared to LD/CD. This natural source of L-dopa may provide advantages in the long-term therapy of PD over standard L-dopa preparations because to its quick absorption and lack of dyskinesias [15]. In a PD rat model, boiled and fermented seed n-propanol extract may be more neuroprotective than fresh seeds [16].
Shankhapushpi (Convolvulus pluricaulis)
CP extract (150 mg/kg) lowered tau protein and mRNA levels in scopolamine-treated rats, along with APP levels. Microscopically, the extract inhibited scopolamine neurotoxicity, demonstrating neuroprotective effects. CP treatment reduced scopolamine's neurotoxic effects, indicating it is neuroprotective [17]. Pretreatment with C. pluricaulis restored antioxidant and apoptotic indicators including SOD, CAT, p53, and caspase-3, reduced reactive oxygen species generation and mitochondrial membrane depolarization. GC-MS study found rich flavonoids and polyphenols in C. pluricaulis [18].
Mandukparni (Centella asiatica)
Open field and water T-maze tests examine locomotion, learning, and memory. Cresyl violet and apoptosis stain neuronal cell morphology. In the same animals' hippocampus, we employed immunohistochemistry to look at the glutamate AMPA receptor GluA1 subunit and the GABA receptor GABAA 1 subunit. 30 mg/kg enhanced comprehension, recollection, and memory consolidation (p 0.05), but had little effect on reversal learning [19]. CAW and several of its constituents stimulate dendritic arborization and synaptic differentiation, which may explain its cognitive effects. Since CAW and its constituent chemicals enhanced neuronal endpoints, the extract may have therapeutic potential beyond Alzheimer's disease [20]. E. alsinoides and C. asiatica are promising in the treatment of inflammatory illnesses, wound healing, and immunomodulatory function. Both herb extracts inhibit AChE and increase visual memory [21].
Haridra (Curcumin)
Curcumin, its isoforms, conjugates, and bio-available forms bind to fibrillar Aß plaques and CAA in post-mortem alzheimer's brain tissue, according to a study. Curcumin may be an excellent alternative for in vivo diagnostics in Alzheimer's disease, such as retinal fluorescence imaging, because conjugates and bio-available Curcumin derivatives have similar binding properties [22]. Curcumin's anti-inflammatory and antioxidant effects help treat developing disorders [23].
Curcumin pharmacology reveals its therapeutic ability and limitations in treating neurodegenerative illnesses and brain cancers [24]. Curcumin possesses anti-amyloidogenic and tau-protein-affecting effects. Some studies suggest Curcumin could prevent and treat neurodegenerative brain disorders [25]. Curcumin nanoparticles and their putative mechanism(s) of action have been defined in CNS illnesses like Parkinson's, Huntington, and Alzheimer's [26].
Curcumin, a TNF blocker in numerous cell types and tissues, is chemically optimised to produce an injectable depot for the continuous local release of Curcumin to treat neuroinflammation. ELPs serve as medication transporters and biomaterials. ELP Curcumin conjugates have high drug loads, fast Curcumin release via degradable carbamate bonds, and bioactivity against TNF-induced cytotoxicity and monocyte activation [12]. Curcumin binds and inhibits the addition of the-sheet conformations of the amyloid characteristic of many neurodegenerative illnesses. It also restores inflammatory systems to balance, boosts thermal impact systems for improved clearing of hazardous aggregates, and scavenges free radicals [20].
Amla (Emblica officinalis)
A study investigated the pharmacological action of Emblica officinalis (EOT) tannins by producing cognitive impairment in animals with a highsalt, High-Cholesterol Diet (HSCD), Emblica tannins bind strongly to Nrf2 receptors in Silicone tests. The model community's changed oxidative stress biomarkers improved rats' Morris water maze performance. EOT supplementation increased Nrf2 in hippocampus and cortical CA1 regions. A new EOT action mechanism (the Nrf2-ARE pathway) can be exploited for cognitive insufficiency therapy [27,28].
Yasti madhu (Glycrhiza glabra)
Glyccrhiza improved motor deficits and cognitive problems in rats with postischemia and middle cerebral artery blockage by suppressing microglia activation and proinflammatory cytokine production (MCAO). In this study, we examined Glycrhiza's effect on Kainic Acid-induced neuronal death (KA). Intracerebroventricular (ICV) KA causes neuronal loss in the hippocampus CA1 and CA3 areas. KA's anti-inflammatory and antiexcittoxic characteristics provide neuroprotection [29].
One study found that V. faba, U. rhyncophylla, and G. glabra water extracts protect HypoE22 cells and isolated rat striatum specimens from 6-hydroxydopamine. Extract activities were investigated with LDH, nitrites, 8-IsO-Prostaglandin (PG) F2, or a pharmacological association therapy. These extracts are efficient in reducing striatal DA turnover and lowering LDH and nitrite levels [30].
Neem (Azdirachtica indica)
AI contains analgesic, antiviral, and antioxidant properties, according to a study. Neuroprotective efficacy of AI extract in sciatic nerve binding animal models; (PSNL). Male Wistar rats had PSNL produced via nerve ligation (180 g to 200 g). Rats were given Pyridoxine (100 mg/kg, p.o.) or AI (100 mg/kg, 200 mg/kg, 400 mg/kg, p.o.) for 28 days. Neuronal and reactive oxygen levels were lowered by AI (200 mg/kg and 400 mg/kg). AI reduces PSNL-mediated histological aberration. Azadirachta indica protects against PSNL-caused neuropathy [31]. Hyperglycemia causes oxidative stress, which makes nerves feel compressed. Oxidative stress hinders nerve regrowth and function. A. indica flower extract's anti-oxidant and anti-diabetic effects helped rats recuperate from a diabetic nerve crush trauma (DM) [32].
Natural products and their separated natural components have neuroprotective, therapeutic, and drug development potential against neurodegenerative disorders. Despite their promising neuroprotective activities against neurodegenerative diseases in preclinical settings, translating promising preclinical neuroprotective research to clinical application has proven challenging. There are no positive results in human clinical trials of neurodegenerative diseases. Low bioavailability and limited water solubility, physicochemical instability, fast metabolism, and capacity to pass blood-brain barrier could impair natural products' therapeutic performance. provide more explanation.
Tea catechins, Resverat, and Curcumin have low bioavailability and limited stability because they are vulnerable to degradation or transformation to inactive derivates. Their effectiveness will suffer. To overcome these issues, the use of nanotechnology and nanocarrier-based approaches in the delivery of natural products and their isolated compounds may help and improve the therapeutic responses and enhance their effectiveness. There incorporation of nanoparticle in the delivery system can increase the bioavailability of natural products and their compounds. The most common types of nanoparticle used are polymeric nanoparticles, nanogels, solid lipid nanoparticle, crystal nanoparticle, liposomes, micelles, and complexes with dendrimers [33]. There have been several studies reported on the use of nanoparticle with natural products and their compound, for example, Epigallocatechin-3-gallate for the treatments of Alzheimer’s disease, rosmarinic acid in the management of Huntington’s disease and Curcumin for brain disease (Tables 1-4).
| Plant extracts/phytochemicals (plant source)/natural products/substances | Study model | Neuroprotective activities | References |
|---|---|---|---|
| Arctigenin extracted from Fructus arctii | Rotenone-induced rats |
|
[34] |
| Apium graveolens L. | MPTP-induced mouse |
|
[35] |
| Agaricus blazei extract | Rotenone-induced mouse |
|
[36,37] |
| Dihydromyricetin (DHM) (a natural flavonoid extracted from Ampelopsis grossedentata) | MPTP-induced mouse |
|
[38] |
| Agaropentaose, agaro-oligosaccharide monomer which is hydrolysates of agarose isolated from red algae | 6-ODHA-induced neurotoxicity in SH-SY5Y cells |
|
[39] |
| Boswellic acids | Rotenone-induced rats |
|
[40] |
| Capsicum annuum L. extract | Rotenone-induced mouse |
|
[41] |
| Coeloglossum viride var. Bracteatum extract | MPTP-induced neurotoxicity in mouse and glutamate-induced excitotoxicity in primary cortical neuron cultures |
|
[42] |
| Curcuminoids (Curcuma longa (L.) rhizomes) | MPTP-induced mouse |
|
[43] |
| β-Caryophyllene, a plant-derived cannabinoid compound known as phytocannabinoid | Rotenone-induced rats |
|
[44] |
| Fish oil supplementation (rich in omega-3 polyunsaturated fatty acids) | 6-OHDA-induced rats |
|
[45] |
| Germinated brown rice | Rotenone-induced rats |
|
[46] |
| Oxalis corniculata extract | MPTP-induced mouse | Improved memory retention and retrieval | [47] |
| Olive leaf extracts (Olea europaea L.) | Rotenone-induced rats |
|
[48] |
| Puerarin (an active component of Pueraria montana var. lobata (willd.) Sanjappa and Pradeep) | MPTP-induced mouse |
|
[49] |
| Rosmarinic acid isolated from callus of Perilla frutescens | 6-OHDA induced rats |
|
[50] |
| Sophora tomentosa extract | MPTP-induced mouse |
|
[51] |
| Tinospora cordifolia ethanol extract | 6-OHDA-induced rats |
|
[52] |
| Tribulus terrestris extract | Rotenone-induced mouse |
|
[53] |
| Ethyl acetate fraction of Urtica dioicalinn | MPTP-induced rats |
|
[54] |
| Zingiber zerumbet (L.) Smith ethyl |
Paraquat-induced rats |
|
[55] |
| Plant extracts/phytochemicals (plant source)/natural products/substances | Study model | Neuroprotective activities | References |
|---|---|---|---|
| Turmeric (powdered rhizome of Curcuma longa Linn (5% Curcumin) | Case studies of 3 patients with progressive dementia | Improvement in the behavioral symptoms and quality of life | [56] |
| Coconut oil enriched Mediterranean diet | 44 patients with Alzheimer’s disease | Improved the cognitive functions | [57] |
| Germinated brown rice (Malaysian mixed varieties; MR219 and MR220) | Aβ (1–42) induced toxicity in SH-SY5Y cells |
|
[58] |
| Huperzine A isolated from Huperzia serrata | Hypoxic-ischemic and glutamate-induced brain injury and cytotoxicity |
|
[59] |
| Huperzine A isolated from Huperzia serrata | 50 patients with Alzheimer’s disease | Improvement in memory, cognitive, and behavior functions | [60] |
| Methanolic extract of Lactuca capensis thunb. leaves | Aβ (1–42) induced neurotoxicity in rats |
|
[61] |
| Osmotin, a plant protein extracted from Nicotiana tabacum | Aβ (1–42) treated mouse and Aβ (1–42) induced neurotoxicity in HT22 cells |
|
[62] |
| Safflower yellow (natural safflower aqueous extract) | Aβ (1–42) induced rats |
|
[63] |
| Tabernaemontana divaricata root extract | Aβ (25–35) induced mouse |
|
[64] |
| Yacon (Smallanthus sonchifolius (poepp and endl) H. Robinson) leaf extract | Aβ (25–35) induced rats |
|
[65] |
| Plant extracts/phytochemicals (plant source)/natural products/substances | Study model | Neuroprotective activities | References |
|---|---|---|---|
| Anthocyanin extracted from strawberries | G93A mutant human SOD1 (hSOD1G93A) mouse model of amyotrophic lateral sclerosis |
|
[66] |
| Alpinia oxyphylla fruit extract | Experimental autoimmune encephalomyelitis mouse model of multiple sclerosis |
|
[67] |
| Isogarcinol extracted from Garcinia mangostana L. mangosteen | Experimental autoimmune encephalomyelitis-induced mouse |
|
[68] |
| Ishige okamurae | Experimental autoimmune encephalomyelitis-induced rats |
|
[69] |
| Nigella sativa | Experimental autoimmune encephalomyelitis-induced rats |
|
[70] |
| Radix Rehmanniae extract | Experimental autoimmune encephalomyelitis-induced mouse |
|
[71] |
| White grape (Vitis vinifera) | Experimental autoimmune encephalomyelitis-induced mouse |
|
[72] |
| Walnut extract | Lipopolysaccharide-induced neurotoxicity in rat microglial cell line |
|
[73] |
| Plant extracts/phytochemicals (plant source)/natural products/substances | Study model | Neuroprotective activities | References |
|---|---|---|---|
| Ethanolic extract of Cocculus laurifolius leaves | Strychnine-induced convulsions in albino rats |
|
[74] |
| Coeloglossum viride var. Bracteatum |
A combination of D-galactose and aluminum chloride-induced aging mouse |
|
[75] |
| Methanolic extract of Cinnamomum camphora leaves | Maximal electroshock-induced seizures in albino Wistar rats |
|
[76] |
| Phragmanthera austroarabica extract | Pentylenetetrazol-kindled mouse |
|
[77] |
| Parawixin 10, a compound isolated from Parawixia bistriata spider venom | A rat excitotoxicity model of brain injury by kainic acid, N-methyl-D-aspartate, and pentylenetetrazol |
|
[78,79] |
| White rose (Rosa hybrida) petal extract | Kainic acid-induced mouse and in HB1.F3 human neural stem cells |
|
[80] |
| Rosemary extract | Kainic acid-induced rats autoimmune encephalomyelitis-induced mouse |
|
[81] |
| Walnut extract | Lipopolysaccharide-induced neurotoxicity in rat microglial cell line |
|
[73] |
Discussion & Conclusion
There is currently an epidemic of Alzheimer's disease as well as other dementias. Symptomatic treatments were not effective in bringing them under control. Tacrine and donepezil are examples of core-acting pharmaceutical drugs that are now being used to increase the quantity of acetylcholine present in the brain. In the treatment of Alzheimer's Disease (AD), numerous herbs with roots in traditional medicine and ayurveda have been studied for their potential therapeutic efficacy. One's approach to the management of AD may benefit from using elements of both traditional Ayurvedic practise and the usage of herbs derived from a variety of geographical locations. In spite of the small number of researches that have been published, it has been demonstrated that all of the bioactive chemicals that have been discussed have a significant neuroprotective impact in animal models of Parkinson's disease. As a consequence of this, bioactive chemicals obtained from natural products have the potential to serve as an important component of Parkinson's disease treatments. The scientific community requires additional methodical research that focuses on discovering active compounds in plants and investigating the mechanisms of action of these active chemicals. This study will be helpful in the conduct of clinical trials to evaluate the efficacy of the highlighted herbal items in the treatment of dementia, and it may also contribute to the development of innovative dementia treatments.
Acknowledgment
Authors are thankful to Shobhit University for providing the internet Facilities as well as access to journal and throughout guidance in carrying out the review paper.
Conflict of Interest
No potential conflicts of interest are declared by the authors.
Ethics Approval
This article does not contain any experiments involving human subjects or animals that were conducted by the author.
References
- Rehman, Muneeb U., Adil F. Wali, Anas Ahmad and Sheeba Shakeel, et al. "Neuroprotective Strategies for Neurological Disorders by Natural Products: An Update." Curr Neuropharmacol 17 (2019): 247-67.
[Crossref] [Google scholar] [Pubmed]
- Venkatesan, Ramu, Eunhee Ji and Sun Yeou Kim. "Phytochemicals that Regulate Neurodegenerative Disease by Targeting Neurotrophins: A Comprehensive Review." Biomed Res Int 2015 (2015): 814068.
[Crossref] [Google scholar] [Pubmed]
- Nishteswar, K., Hemang Joshi and Rahul Dutt Karra. "Role of Indigenous Herbs in the Management of Alzheimer's Disease." Anc Sci Life 34 (2014): 3.
[Crossref] [Google scholar] [Pubmed]
- Mishra, Rachana, Shaffi Manchanda, Muskan Gupta and Taranjeet Kaur, et al. "Tinospora Cordifolia Ameliorates Anxiety-like Behavior and Improves Cognitive Functions in Acute Sleep Deprived Rats." Sci Rep 6 (2016): 1-15.
[Crossref] [Google scholar] [Pubmed]
- Singh, Surabhi and Madhu G. Tapadia. "Molecular Basis for Efficacy of Guduchi and Madhuyashti Feeding on different Environmental Stressors in Drosophila." Cell Stress Chaperones 24 (2019): 549-65.
[Crossref] [Google scholar] [Pubmed]
- Sharma, Anuradha and Gurcharan Kaur. "Tinospora Cordifolia as a Potential Neuroregenerative Candidate against Glutamate induced Excitotoxicity: An In Vitro Perspective." BMC Complement Altern Med 18 (2018): 1-17.
[Crossref] [Google scholar] [Pubmed]
- Malve, Harshad Onkarrao. "Exploring Bhavana Samskara using Tinospora Cordifolia and Phyllanthus Emblica Combination for Learning and Memory in Mice." J Ayurveda Integr Med 6 (2015): 233-40.
[Crossref] [Google scholar] [Pubmed]
- Kosaraju, Jayasankar, Santhivardhan Chinni, Partha Deb Roy and Elango Kannan, et al. "Neuroprotective Effect of Tinospora Cordifolia Ethanol Extract on 6-Hydroxy Dopamine induced Parkinsonism." Indian J Pharmacol 46 (2014): 176-80.
[Crossref] [Google scholar] [Pubmed]
- Lieu, Christopher A., Kala Venkiteswaran, Timothy P. Gilmour and Anand N. Rao, et al. "The Antiparkinsonian and Antidyskinetic Mechanisms of Mucuna Pruriens in the MPTP-Treated Nonhuman Primate." Evid Based Complement Alternat Med 2012 (2012): 840247.
[Crossref] [Google scholar] [Pubmed]
- Rai, Sachchida N., Hareram Birla, Saumitra S. Singh and Walia Zahra, et al. "Mucuna Pruriens Protects against MPTP Intoxicated Neuroinflammation in Parkinson’s Disease through NF-κB/pAKT Signaling Pathways." Front Aging Neurosci 9 (2017): 421.
[Crossref] [Google scholar] [Pubmed]
- Cilia, Roberto, Janeth Laguna, Erica Cassani and Emanuele Cereda, et al. "Mucuna Pruriens in Parkinson Disease: A Double-blind, Randomized, Controlled, Crossover Study." Neurology 89 (2017): 432-8.
[Crossref] [Google scholar] [Pubmed]
- Sloane, A. H. and M. Misuraca. Genetic Changes. Amsterdam: Elsevier, Netherlands, (2013).
- Johnson, Shelby L., Hyun Y. Park, Nicholas A. DaSilva and Dhiraj A. Vattem, et al. "Levodopa-Reduced Mucuna Pruriens Seed Extract shows Neuroprotective effects against Parkinson’s Disease in Murine Microglia and Human Neuroblastoma Cells, Caenorhabditis Elegans, and Drosophila Melanogaster." Nutrients 10 (2018): 1139.
[Crossref] [Google scholar] [Pubmed]
- Poddighe, Simone, Francescaelena de Rose, Roberto Marotta and Roberta Ruffilli, et al. "Mucuna Pruriens (Velvet Bean) Rescues Motor, Olfactory, Mitochondrial and Synaptic Impairment in PINK1B9 Drosophila Melanogaster Genetic Model of Parkinson’s Disease." PLoS One 9 (2014): e110802.
[Crossref] [Google scholar] [Pubmed]
- Katzenschlager, Regina, Alan Evans, A. Manson and P. N. Patsalos, et al. "Mucuna Pruriens in Parkinson’s Disease: A Double Blind Clinical and Pharmacological Study." J Neurol Neurosurg Psychiatry 75 (2004): 1672-7.
[Crossref] [Google scholar] [Pubmed]
- Adi, Yosua Kristian, Rini Widayanti and Tri Wahyu Pangestiningsih. "n-Propanol Extract of Boiled and Fermented Koro Benguk (Mucuna Pruriens Seed) Shows a Neuroprotective Effect in Paraquat Dichloride-induced Parkinson’s Disease Rat Model." Vet World 11 (2018): 1250-4.
[Crossref] [Google scholar] [Pubmed]
- Bihaqi, Syed Waseem, Avninder Pal Singh and Manisha Tiwari. "Supplementation of Convolvulus Pluricaulis attenuates Scopolamine-induced Increased Tau and Amyloid Precursor Protein (AβPP) Expression in Rat Brain." Indian J Pharmacol 44 (2012): 593-8.
[Crossref] [Google scholar] [Pubmed]
- Rachitha, P., K. Krupashree, G. V. Jayashree and Hemanth Kumar Kandikattu, et al. "Chemical Composition, Antioxidant Potential, Macromolecule Damage and Neuroprotective Activity of Convolvulus Pluricaulis." J Tradit Complement Med 8 (2018): 483-96.
[Crossref] [Google scholar] [Pubmed]
- Binti Mohd Yusuf Yeo, Nor Aqilah, Sangu Muthuraju, Jia Hui Wong and Faruque Reza Mohammed, et al. "Hippocampal Amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic Acid GluA1 (AMPA GluA1) Receptor Subunit involves in Learning and Memory Improvement following Treatment with Centella Asiatica Extract in Adolescent Rats." Brain Behav 8 (2018): e01093.
[Crossref] [Google scholar] [Pubmed]
- Shannon, Kate. Myocardial Extraction from Neonatal Rats. Amsterdam: Elsevier, Netherlands, (2016).
- Yadav, Mukesh Kumar, Santosh Kumar Singh, Manish Singh and Shashank Shekhar Mishra, et al. "Neuroprotective activity of Evolvulus Alsinoides and Centella Asiatica Ethanolic Extracts in Scopolamine-induced Amnesia in Swiss Albino Mice." Open Access Maced J Med Sci 7 (2019): 1059-66.
[Crossref] [Google scholar] [Pubmed]
- Den Haan, Jurre, Tjado HJ Morrema, Annemieke J. Rozemuller and Femke H. Bouwman, et al. "Different Curcumin forms Selectively Bind Fibrillar Amyloid Beta in Post Mortem Alzheimer’s Disease Brains: Implications for In Vivo Diagnostics." Acta Neuropathol Commun 6 (2018): 1-12.
[Crossref] [Google scholar] [Pubmed]
- Del Prado-Audelo, María L., Isaac H. Caballero-Florán, Jorge A. Meza-Toledo and Néstor Mendoza-Muñoz, et al. "Formulations of Curcumin Nanoparticles for Brain Diseases." Biomolecules 9 (2019): 56.
[Crossref] [Google scholar] [Pubmed]
- Lee, Wing-Hin, Ching-Yee Loo, Mary Bebawy and Frederick Luk, et al. "Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century." Curr Neuropharmacol 11 (2013): 338-78.
[Crossref] [Google scholar] [Pubmed]
- Pluta, Ryszard, Marzena Ułamek-Kozioł and Stanisław J. Czuczwar. "Neuroprotective and Neurological/Cognitive Enhancement effects of Curcumin after Brain Ischemia Injury with Alzheimer’s Disease Phenotype." Int J Mol Sci 19 (2018): 4002.
[Crossref] [Google scholar] [Pubmed]
- Yavarpour-Bali, Hanie, Maryam Ghasemi-Kasman and Marzieh Pirzadeh. "Curcumin-Loaded Nanoparticles: A Novel Therapeutic Strategy in Treatment of Central Nervous System Disorders." Int J Nanomedicine 14 (2019): 4449-60.
[Crossref] [Google scholar] [Pubmed]
- Husain, Ibraheem, Mohd Akhtar, Tushar Madaan and Divya Vohora, et al. "Tannins Enriched Fraction of Emblica Officinalis Fruits Alleviates High-Salt and Cholesterol Diet-induced Cognitive Impairment in Rats via Nrf2–ARE Pathway." Front Pharmacol 9 (2018): 23.
[Crossref] [Google scholar] [Pubmed]
- Puppala, Muthenna, Jessica Ponder, Palla Suryanarayana and Geereddy Bhanuprakash Reddy, et al. "The Isolation and Characterization of β-Glucogallin as a Novel Aldose Reductase Inhibitor from Emblica Officinalis." PloS One 7 (2012): e31399.
[Crossref] [Google scholar] [Pubmed]
- Lee, Jiyeon, Eunjin Lim, Yumi Kim and Endan Li, et al. "Ghrelin Attenuates Kainic Acid-induced Neuronal Cell Death in the Mouse Hippocampus." J Endocrinol 205 (2010): 263-70.
[Crossref] [Google scholar] [Pubmed]
- Orlando, Giustino, Annalisa Chiavaroli, Sheila Leone and Luigi Brunetti, et al. "Inhibitory Effects Induced by Vicia Faba, Uncaria Rhyncophylla, and Glycyrrhiza Glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine." Antioxidants 8 (2019): 602.
[Crossref] [Google scholar] [Pubmed]
- Kandhare, Amit D., Anwesha A. Mukherjee and Subhash L. Bodhankar. "Neuroprotective Effect of Azadirachta Indica Standardized Extract in Partial Sciatic Nerve Injury in Rats: Evidence from Anti-Inflammatory, Antioxidant and Anti-Apoptotic Studies." EXCLI J 16 (2017): 546-65.
[Crossref] [Google scholar] [Pubmed]
- Sriraksa, Napatr, Ratchaniporn Kongsui, Sitthisak Thongrong and Acharaporn Duangjai, et al. "Effect of Azadirachta Indica Flower Extract on Functional Recovery of Sciatic Nerve Crush Injury in Rat Models of DM." Exp Ther Med 17 (2019): 541-50.
[Crossref] [Google scholar] [Pubmed]
- Mohd Sairazi, Nur Shafika and K. N. S. Sirajudeen. "Natural Products and their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases." Evid Based Complement Alternat Med 2020 (2020): 6565396.
[Crossref] [Google scholar] [Pubmed]
- Zhang, Na, Deqiang Dou, Xiaoku Ran and Tingguo Kang. "Neuroprotective Effect of Arctigenin against Neuroinflammation and Oxidative Stress induced by Rotenone." RSC Adv 8 (2018): 2280-92.
[Crossref] [Google scholar] [Pubmed]
- Chonpathompikunlert, Pennapa, Phetcharat Boonruamkaew, Wanida Sukketsiri and Pilaiwanwadee Hutamekalin, et al. "The Antioxidant and Neurochemical Activity of Apium Graveolens L. and its Ameliorative effect on MPTP-induced Parkinson-like Symptoms in Mice." BMC Complement Altern Med 18 (2018): 1-12.
[Crossref] [Google scholar] [Pubmed]
- Venkatesh Gobi, Veerappan, Srinavasagam Rajasankar, William Moses Swaminathan Johnson, and Kaliyaperumal Prabu, et al. "Neuroprotective effect of Agaricus Blazei Extract against Rotenone-induced Motor and Nonmotor Symptoms in Experimental Model of Parkinson’s Disease." Int J Nutr Pharmacol Neurol Dis 8 (2018): 59-65.
- Venkatesh Gobi, Veerappan, Srinivasagam Rajasankar, Muthu Ramkumar and Chinnasamy Dhanalakshmi, et al. "Agaricus Blazei Extract Abrogates Rotenone-induced Dopamine Depletion and Motor Deficits by its Anti-Oxidative and Anti-Inflammatory Properties in Parkinsonic Mice." Nutr Neurosci 21 (2018): 657-66.
[Crossref] [Google scholar] [Pubmed]
- Ren, Zhao-Xiang, Ya-fei Zhao, Ting Cao, and Xue-chu Zhen. "Dihydromyricetin Protects Neurons in an MPTP-induced Model of Parkinson's Disease by Suppressing Glycogen Synthase Kinase-3 Beta Activity." Acta Pharmacol Sin 37 (2016): 1315-24.
[Crossref] [Google scholar] [Pubmed]
- Ye, Qiang, Wei Wang, Cui Hao and Xiangzhao Mao. "Agaropentaose Protects SH-SY5Y Cells against 6-Hydroxydopamine-induced Neurotoxicity through Modulating NF-κB and p38MAPK Signaling Pathways." J Functional Foods 57 (2019): 222-32.
- Ameen, Angie M., Amany Y. Elkazaz, Hala MF Mohammad and Bassant M. Barakat. "Anti-Inflammatory and Neuroprotective activity of Boswellic Acids in Rotenone Parkinsonian Rats." Can J Physiol Pharmacol 95 (2017): 819-29.
[Crossref] [Google scholar] [Pubmed]
- Abdel-Salam, Omar ME, Amany A. Sleem, Eman R. Youness and Noha N. Yassen, et al. "Capsicum Protects against Rotenone-induced Toxicity in Mice Brain via reduced Oxidative Stress and 5-Lipoxygenase Activation." J Pharm Pharmacol Res 2 (2018): 60-77.
- Pan, Rui-Yuan, Jun Ma, Huan-Tong Wu and Qing-Shan Liu, et al. "Neuroprotective Effects of a Coeloglossum Viride var. Bracteatum extract in vitro and in vivo." Sci Rep 7 (2017): 1-9.
[Crossref] [Google scholar] [Pubmed]
- Ojha, Rudra P., Manisha Rastogi, B. Parimala Devi and Aruna Agrawal, et al. "Neuroprotective effect of Curcuminoids against Inflammation-Mediated Dopaminergic Neurodegeneration in the MPTP Model of Parkinson’s Disease." J Neuroimmune Pharmacol 7 (2012): 609-18.
[Crossref] [Google scholar] [Pubmed]
- Ojha, Shreesh, Hayate Javed, Sheikh Azimullah and M. Emdadul Haque. "β-Caryophyllene, a Phytocannabinoid attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease." Mol Cell Biochem 418 (2016): 59-70.
[Crossref] [Google scholar] [Pubmed]
- Mori, Marco Aurélio, Ana Marcia Delattre, Bruno Carabelli and Claudia Pudell, et al. "Neuroprotective effect of Omega-3 Polyunsaturated Fatty Acids in the 6-OHDA Model of Parkinson's Disease is Mediated by a Reduction of Inducible Nitric Oxide Synthase." Nutr Neurosci 21 (2018): 341-51.
[Crossref] [Google scholar] [Pubmed]
- Chompoopong, Supin, Sunit Jarungjitaree, Tideeporn Punbanlaem and Thanaporn Rungruang, et al. "Neuroprotective Effects of Germinated Brown Rice in Rotenone-induced Parkinson’s-like Disease Rats." Neuromolecular Med 18 (2016): 334-46.
[Crossref] [Google scholar] [Pubmed]
- Aruna, K., P. Devi Raja Rajeswari and S. Raja Sankar. "The Effect of Oxalis Corniculata Extract against the Behavioral Changes induced by 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) in Mice." J App Pharm Sci 7 (2017): 148-53.
- Sarbishegi, Maryam, Enam Alhagh Charkhat Gorgich, Ozra Khajavi and Gholamreza Komeili, et al. "The Neuroprotective Effects of Hydro-Alcoholic Extract of Olive (Olea Europaea L.) Leaf on Rotenone-induced Parkinson’s Disease in Rat." Metab Brain Dis 33 (2018): 79-88.
[Crossref] [Google scholar] [Pubmed]
- Zhu, Guoqi, Xuncui Wang, Shengbing Wu and Xiaoxiang Li, et al. "Neuroprotective Effects of Puerarin on 1‐methyl‐4‐phenyl‐1,2,3,6‐Tetrahydropyridine induced Parkinson's Disease Model in Mice." Phytother Res 28 (2014): 179-86.
[Crossref] [Google scholar] [Pubmed]
- Wang, Jieyu, Huamin Xu, Hong Jiang and Xixun Du, et al. "Neurorescue Effect of Rosmarinic Acid on 6-Hydroxydopamine-lesioned Nigral Dopamine Neurons in Rat Model of Parkinson's Disease." J Mol Neurosci 47 (2012): 113-9.
[Crossref] [Google scholar] [Pubmed]
- Chang, Hung-Chi, Keng-Fan Liu, Chia-Jen Teng and Shu-Chen Lai, et al. "Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress." Nutrients 11 (2019): 252.
[Crossref] [Google scholar] [Pubmed]
- Kosaraju, Jayasankar, Santhivardhan Chinni, Partha Deb Roy and Elango Kannan, et al. "Neuroprotective Effect of Tinospora Cordifolia Ethanol Extract on 6-Hydroxy Dopamine Induced Parkinsonism." Indian J Pharmacol 46 (2014): 176-80.
[Crossref] [Google scholar] [Pubmed]
- Alzahrani, S., W. Ezzat, R. E. Elshaer and A. S. Abd El-Lateef, et al. "Standarized Tribulus Terrestris Extract Protects against Rotenone-induced Oxidative Damage and Nigral Dopamine Neuronal Loss in Mice." J Physiol Pharmacol 69 (2018): 979-94.
[Crossref] [Google scholar] [Pubmed]
- Bisht, Rohit, Bhuwan Chandra Joshi, Ajudhiya Nath Kalia and Atish Prakash. "Antioxidant-Rich Fraction of Urtica Dioica Mediated Rescue of Striatal Mito-Oxidative Damage in MPTP-Induced Behavioral, Cellular, and Neurochemical Alterations in Rats." Mol Neurobiol 54 (2017): 5632-45.
[Crossref] [Google scholar] [Pubmed]
- Ibrahim, Farah Wahida, U. Noraashikin Zainudin, M. Abdul Latif and Asmah Hamid. "Neuroprotective Effects of Ethyl Acetate Extract of Zingiber Zerumbet (L.) Smith against Oxidative Stress on Paraquat-induced Parkinsonism in Rats." Sains Malaysiana 47 (2018): 2337-47.
- Hishikawa, Nozomi, Yoriko Takahashi, Yoshinobu Amakusa and Yuhei Tanno, et al. "Effects of Turmeric on Alzheimer's Disease with Behavioral and Psychological Symptoms of Dementia." Ayu 33 (2012): 499-504.
[Crossref] [Google scholar] [Pubmed]
- de la Rubia Ortí, José Enrique, María Pilar García-Pardo, Eraci Drehmer and David Sancho Cantus, et al. "Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study." J Alzheimers Dis 65 (2018): 577-87.
[Crossref] [Google scholar] [Pubmed]
- Azmi, Nur Hanisah, Maznah Ismail, Norsharina Ismail and Mustapha Umar Imam, et al. "Germinated Brown Rice Alters Aβ (1-42) Aggregation and Modulates Alzheimer’s Disease-related Genes in Differentiated Human SH-SY5Y Cells." Evid Based Complement Alternat Med 2015 (2015): 153684.
[Crossref] [Google scholar] [Pubmed]
- Wang, Rui and Xi Can Tang. "Neuroprotective Effects of Huperzine A. A Natural Cholinesterase Inhibitor for the Treatment of Alzheimer's Disease" Neurosignals 14 (2005): 71-82.
[Crossref] [Google scholar] [Pubmed]
- Xu, Si-Sun, Z. X. Gao, Zheng Weng and Z. M. Du, et al. "Efficacy of Tablet Huperzine-A on Memory, Cognition, and Behavior in Alzheimer's Disease." Zhongguo Yao Li Xue Bao 16 (1995): 391-5.
- Postu, Paula Alexandra, Jaures AK Noumedem, Oana Cioanca and Monica Hancianu, et al. "Lactuca Capensis Reverses Memory Deficits in Aβ1‐42‐induced an Animal Model of Alzheimer's Disease." J Cell Mol Med 22 (2018): 111-22.
[Crossref] [Google scholar] [Pubmed]
- Ali, Tahir, Gwang Ho Yoon, Shahid Ali Shah and Hae Young Lee, et al. "Osmotin Attenuates Amyloid Beta-induced Memory Impairment, Tau Phosphorylation and Neurodegeneration in the Mouse Hippocampus." Sci Rep 5 (2015): 1-17.
[Crossref] [Google scholar] [Pubmed]
- Zhang, Lu, Zhangjiuzhi Zhou, Wei Zhai and Jie Pang, et al. "Safflower Yellow Attenuates Learning and Memory Deficits in Amyloid β-Induced Alzheimer’s Disease Rats by Inhibiting Neuroglia Cell Activation and Inflammatory Signaling Pathways." Metab Brain Dis 34 (2019): 927-39.
[Crossref] [Google scholar] [Pubmed]
- Khongsombat, Onrawee, Walika Nakdook, Kornkanok Ingkaninan. "Inhibitory Effects of Tabernaemontana Divaricata Root Extract on Oxidative Stress and Neuronal Loss Induced by Amyloid β25–35 Peptide in Mice." J Tradit Complement Med 8 (2018): 184-9.
[Crossref] [Google scholar] [Pubmed]
- Martinez-Oliveira, Patrícia, Micaela Federizzi de Oliveira, Niége Alves and Ritiéle Pinto Coelho, et al. "Yacon Leaf Extract Supplementation Demonstrates Neuroprotective Effect against Memory Deficit related to β-amyloid-induced Neurotoxicity." J Functional Foods 48 (2018): 665-75.
- Winter, Aimee N., Erika K. Ross, Heather M. Wilkins and Trisha R. Stankiewicz, et al. "An Anthocyanin-Enriched Extract from Strawberries Delays Disease Onset and Extends Survival in the hSOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis." Nutr Neurosci 21 (2018): 414-26.
[Crossref] [Google scholar] [Pubmed]
- Huang, Kuo-Kuei, Meng-Nan Lin, Yi-Ling Hsu and I. Lu, et al. "Alpinia Oxyphylla Fruit Extract Ameliorates Experimental Autoimmune Encephalomyelitis through the Regulation of Th1/Th17 Cells." Evid Based Complement Alternat Med 2019 (2019): 6797030.
[Crossref] [Google scholar] [Pubmed]
- Wang, Mengqi, Yufei Xie, Youxiu Zhong and Juren Cen, et al. "Amelioration of Experimental Autoimmune Encephalomyelitis by Isogarcinol Extracted from Garcinia Mangostana L. Mangosteen." J Agric Food Chem 64 (2016): 9012-21.
[Crossref] [Google scholar] [Pubmed]
- Ahn, Meejung, Jeongtae Kim, Wonjun Yang and Yuna Choi, et al. "Amelioration of Experimental Autoimmune Encephalomyelitis by Ishige Okamurae." Anat Cell Biol 51 (2018): 292-8.
[Crossref] [Google scholar] [Pubmed]
- Noor, Neveen A., Heba M. Fahmy, Faten F. Mohammed and Anwar A. Elsayed, et al. "Nigella Sativa Amliorates Inflammation and Demyelination in the Experimental Autoimmune Encephalomyelitis-induced Wistar Rats." Int J Clin Exp Pathol 8 (2015): 6269-86.
[Google scholar] [Pubmed]
- Li, Wenting, Hao Wu, Chong Gao and Dan Yang, et al. "Radix Rehmanniae Extract Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing Macrophage-Derived Nitrative Damage." Front Physiol 9 (2018): 864.
[Crossref] [Google scholar] [Pubmed]
- Giacoppo, Sabrina, Maria Galuppo, Giovanni Enrico Lombardo and Maria Malgorzata Ulaszewska, et al. "Neuroprotective Effects of a Polyphenolic White Grape Juice Extract in a Mouse Model of Experimental Autoimmune Encephalomyelitis." Fitoterapia 103 (2015): 171-86.
[Crossref] [Google scholar] [Pubmed]
- Thangthaeng, Nopporn, Shibu M. Poulose, Derek R. Fisher and Barbara Shukitt-Hale. "Walnut Extract Modulates Activation of Microglia through Alteration in Intracellular Calcium Concentration." Nutr Res 49 (2018): 88-95.
[Crossref] [Google scholar] [Pubmed]
- Maqbool, Sidra, Ishrat Younus, Rafia Sadaf and Anab Fatima. "Neuro-Pharmacological Evaluation of Anticonvulsant and Neuroprotective Activity of Cocculus Laurifolius Leaves in Wistar Rats." Metab Brain Dis 34 (2019): 991-9.
[Crossref] [Google scholar] [Pubmed]
- Zhong, Si-Jia, Lin Wang, Huan-Tong Wu and Rongfeng Lan, et al. "Coeloglossum Viride var. Bracteatum Extract Improves Learning and Memory of Chemically-induced Aging Mice through Upregulating Neurotrophins BDNF and FGF2 and Sequestering Neuroinflammation." J Functional Foods 57 (2019): 40-47.
- Jawaid, Talha, Mehnaz Kamal, Richa Singh and Deepa Shukla, et al. "Anticonvulsant and Neuroprotective effects of Methanolic Extract of Cinnamomum Camphora Leaves in Rat Brain." Orient Pharm Exp Med 18 (2018): 237-46.
- Aldawsari, Hibah M., Basma G. Eid, Thikrayat Neamatallah and Sawsan A. Zaitone, et al. "Anticonvulsant and Neuroprotective Activities of Phragmanthera Austroarabica Extract in Pentylenetetrazole-Kindled Mice." Evid Based Complement Alternat Med 2017 (2017): 5148219.
[Crossref] [Google scholar] [Pubmed]
- Fachim, Helene Aparecida, Marcia Renata Mortari, Leonardo Gobbo-Netto and Wagner Ferreira Dos Santos. "Neuroprotective Activity of Parawixin 10, a Compound Isolated from Parawixia Bistriata Spider Venom (Araneidae: Araneae) in Rats Undergoing Intrahippocampal NMDA Microinjection." Pharmacogn Mag 11 (2015): 579.
[Crossref] [Google scholar] [Pubmed]
- Fachim, Helene Aparecida, Alexandra Olimpio Siqueira Cunha, Adriana Colsera Pereira and René Oliveira Beleboni, et al. "Neurobiological Activity of Parawixin 10, a Novel Anticonvulsant Compound Isolated from Parawixia Bistriata Spider Venom (Araneidae: Araneae)." Epilepsy Behav 22 (2011): 158-64.
[Crossref] [Google scholar] [Pubmed]
- Yon, Jung-Min, Yun-Bae Kim and Dongsun Park. "The Ethanol Fraction of White Rose Petal Extract Abrogates Excitotoxicity-Induced Neuronal Damage in vivo and in vitro through Inhibition of Oxidative Stress and Proinflammation." Nutrients 10 (2018): 1375.
[Crossref] [Google scholar] [Pubmed]
- Naderali, Elahe, Farnaz Nikbakht, Sattar Norouzi Ofogh and Homa Rasoolijazi. "The Role of Rosemary Extract in Degeneration of Hippocampal Neurons induced by Kainic Acid in the Rat: A Behavioral and Histochemical Approach." J Integr Neurosci 17 (2018): 31-43.
[Crossref] [Google scholar] [Pubmed]
Citation: Dubey, Anubhav, Niladry Sekhar Ghosh, Nidhee Agnihotri and Amit Kumar et al. “Herbs Derived Bioactive Compounds and their Potential for the Treatment of Neurological Disorders.” Clin Schizophr Relat Psychoses 16 (2022). Doi: 10.3371/CSRP.DANG.081922.
Copyright: © 2022 Dubey A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.