Research - Clinical Schizophrenia & Related Psychoses ( 2021) Volume 0, Issue 0
Antifungal Activity of the Secondary Metabolites Extracted from Carthamus tinctorius L. against Aspergillus Species Isolated from Stored Medicinal Plants Seeds in the Iraqi Markets
Arabia Yaqoub Hussain1, Hussein J. Hussein2* and Abeer Fauzi Al-Rubaye22Departmnent of Biology, University of Babylon, Babylon, Iraq
Hussein J. Hussein, Departmnent of Biology, University of Babylon, Babylon, Iraq, Email: huss2010feb@yahoo.com
Received: 22-Jul-2021 Accepted Date: Aug 05, 2021 ; Published: 12-Aug-2021
Abstract
The present study, was conducted to investigate the effect of the crude alkaloids, flavonoids, and terpenoids compounds extract from seeds of (Carthamus tinctorius L.) against Aspergillus species isolated from stored medicinal plants seeds collected from different local markets in the province of Babil 2020 in Iraq. Antifungal activity was achieved in vitro by using food poisoning method against Aspergillus species by preparing three concentrations for each crude compound (5,10 and 20) mg/ml and compared with positive control represented by fungicide Quinoleine 50% and negative control represented by 10% dimethyl sulfoxide. The aim of this study was to control of Aspergillus species isolated form stored medicinal plants seeds by using secondary metabolites extracted from seeds of Carthamus tinctorius L. The data collected from the study revealed that, the crude alkaloids, flavonoids, and terpenoids compounds extract from seeds of (Carthamus tinctorius L.) showed significant reduction at P ≤ 0.05 in the growth of Aspergillus species especially at 20 mg/ml compared with negative control. Finally, it can be concluded that Carthamus tinctorius L. is most effective in controlling Aspergillus species, especially terpenoids compounds.
Keywords
Alkaloids • Flavonoids • Terpenoids • Carthamus tinctorius L. • Antifungal
Introduction
Carthamus tinctorius L. (Safflower) of family Asteraceae is a medicinal plant with great potential. Its extract and oil have many therapeutic uses and having great pharmacological importance. Safflower is mainly cultivated for its seeds, oil and flowers. It is to cure many day-to-day ailments, and has proved importance as purgative, analgesic, anti-inflammatory, antipyretic, menstrual problems, post-partum hemorrhage, osteoporosis, diabetes, hepatoprotection, cancer, fibrosis and antioxidant. Carthamine, hydroxyl safflower yellow-A, carthamidine, luteolin are the main phytoactive principles of this plant. Flavonoid derivatives and Furanocoumarins are a target for inflammatory, antimicrobial, anticancer, antidiarrheal drugs [1]. Carthamus tinctorius L. commonly known as Safflower or false saffron is a thistle like, self-compatible, annual, diploid (2n=24) herbaceous crop that thrives in hot and dry climates and is believed to have been domesticated somewhere in the Fertile Crescent region over 4,000 years ago [2]. Carthamus species probably originate from Southern Asia and is known to have been cultivated in China, India, Iran and Egypt almost from prehistoric times. During middle ages it was cultivated in Italy, France, and Spain, and was introduced into United States in 1925 from the Mediterranean region [3]. More than 200 compounds have been isolated from C. tinctorius and the commonly known ones are flavonoids, phenylethanoid glycosides, coumarins, fatty acids, steroids and polysaccharides [4]. Oil content of the seeds is similar to that of olive and includes linoleic acid (63%-72%), oleic acid (16%-25%) and (1%-6%) of linolenic acid [5]. Phytochemical are secondary plants metabolites includes (a) Alkaloids, having the characteristics of antispasmodic, antimalarial, analgesic, diuretic activities, (b)Terpenoids, having the properties of antiviral, anthelmintic, antibacterial, anticancer, antimalarial, anti-inflammatory ,(c) Glycosides are known for its antifungal and antibacterial properties, (d) Phenols and flavonoids are reported to have an antioxidant, anti-allergic, antibacterial properties etc. and (e) Saponins have the properties of anti-inflammatory, antiviral, plant defence activities [6,7]. The resistance of pathogenic fungi to antifungal drugs is one of the major public health problems. Plant extracts have shown inhibitory effect on the growth of wide range of fungi. They are represented a good alternative for prevention and treatment of fungal diseases [8]. From this standpoint, humans should search for natural sources that are less harmful and environmentally friendly in order to control fungi and reduce as much as possible the use of fungicides and pesticides. However, the aim of this study was to control of Aspergillus species isolated form medicinal plants seeds by using secondary metabolites extracted from Carthamus tinctorius L. seeds.
Materials and Methods
Plant material
Safflower seeds (Carthamus tinctorius L), had been purchased from local markets, identified based on the taxonomic features in Iraqi Flora (Table 1) [9]. Seeds of these plants were cleaned, dried, and kept according to Figure 1 [10].
| Scientific name | Local name | English name | Family | Active part used |
|---|---|---|---|---|
| Carthamus tinctorius L. | Asfur, Asfoor, Usfur | Safflower | Asteraceae | Seeds |
Table 1: Scientific, Local, English name, Family, and active parts.
Extraction of the crude alkaloid compounds
Crude Alkaloid compounds were extracted according to Harborne [11].
Extraction of the crude flavonoid compounds
Crude flavonoid compounds were extracted according to Boham [12] .
Extraction of the crude terpenoid compounds
Crude flavonoid compounds were extracted according to Boham [12].
Extraction of the crude terpenoid compounds
Crude terpenoids compounds were extracted according to Harborne [13]. Stock solution of 200 mg/ml for Alkaloid, Flavonoid, and Terpenoid were prepared in 10% Dimethyl Sulfoxide (DMSO) then sterilized by Millipore filter (0.22 μm) and stored at (-20°C) until use [14].
Medicinal plants seeds collection
Medicinal plants seeds were collected from different regions of local markets in the province of Babil/Hillah City 2020 in Iraq.
Isolation and diagnosis of Aspergillus species
To isolate the Aspergillus fungus 100 seeds were taken randomly from each of the collected samples. It was sterilized using 2% sodium chlorate for 2 minutes and then washed with sterile distilled water twice to remove traces of sterile material and dried with sterile filter paper. It was transferred with sterile forceps to 9 cm petri dishes containing 20 ml of pre-prepared Potato Dextrose Agar(PDA) with (chloramphenicol) 50 mg/l to prevent bacterial growth [15], by 5 seeds per dish and three replicates per sample and then incubated in 25°C for 5-7 days. The fungi associated with the seeds were then purified by secondary cultures for identification. Isolated fungi were then diagnosed based on the taxonomic keys of both [16,17]. The fungal isolates were kept in clean, sterile glass containers containing the Nutrient Agar. Containers were incubated at 25°C for a week and then placed in the refrigerator at 4°C until it was used.
Antifungal activity assay of extract
PDA medium was prepared and autoclaved after that a known volume (2 ml) of the each plant extracts is placed in the center of the petri dishes and complete the volume to 20 ml with PDA medium to obtain the required final concentrations (5,10 and 20 mg/ml) of the medicinal plants after complete solidification of the medium, 5 mm disc of seven days old culture of the test fungus were placed aseptically in the center of the Petri plates and incubated at 25 ± 2°C for seven days, simultaneously 0.02 ml of antibiotic solution was added to each assay plate to check the bacterial contamination as suggested [18]. Fungicide Quinoleine 50% was used as positive control and dimethyl sulfoxide as negative control. Observations were recorded on seventh day. The colony diameter was recorded in terms of millimeters. PDA medium devoid of extract served as control. For each treatment three replicates were maintained. The fungi toxicity of extracts was calculated in terms of percent inhibition of mycelia growth by using the formula [19].
Percent Inhibition=(dc-dt/dc) × 100
where:
dc=Average increase in mycelia growth in control.
dt=Average increase in mycelia growth in treatment.
Statistical analysis
All data of treatments were dictated by three replicates. Data were subjected to an analysis of variance by using SPSS 16.0 program, a completely randomized design was used and Least Significant Difference (L.S.D) was performed at P ≤ 0.05.
Results
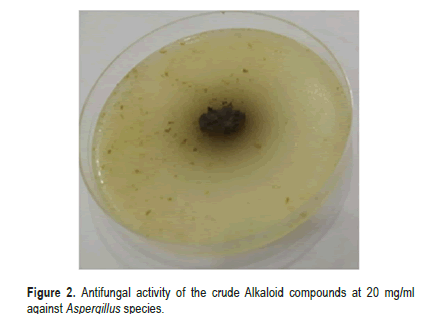
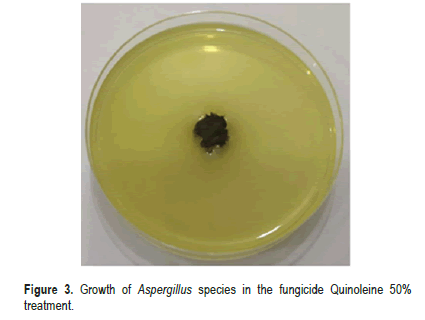
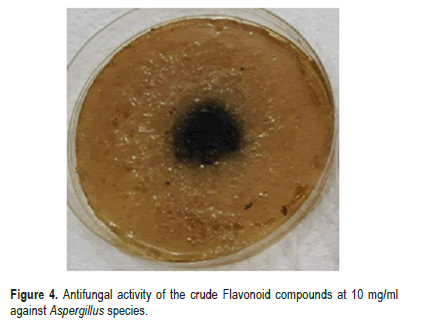
The results of antifungal activity of the crude Alkaloid compounds extracted from the seeds of (Carthamus tinctorius L.) against Aspergillusspecies isolated from stored medicinal plants seeds are presented in Table 2. The antifungal activity of Alkaloid secondary metabolites with three concentration (5,10 and 20 mg/ml) was screened by food poisoning methods. The results revealed that, the crude Alkaloid compounds extracted from the seeds of (Carthamus tinctorius L.) showed significant reduction at P ≤ 0.05 in the growth of Aspergillus species. Antifungal activity was applied at (5,10 and 20) mg/ml. mycelial inhibition ranging from (34.666% in 5 mg/ml, 78% in 10 mg/ ml, and 95% in 20 mg/ ml) compared with negative control and positive fungicide Quinoleine 50% control where inhibition percentage was 0.00% for negative control and 100% for positive control (Figures 2 and 3). On the other hand, the crude flavonoid compounds showed 71% mycelial inhibition at (5 mg/ml) and 88.16% at (10 mg/ ml), and 91% at (20 mg/ml) concentration (Table 3). Thus, it differed significantly compared to the control treatment (Figures 4 and 5).
| Concentrations (mg/ml) | Alkaloids compounds |
|---|---|
| Inhibition percentage % | |
| Negative Control | 0 ± 0.00 |
| 5 mg/ml | 34.666 ± 1.52 |
| 10 mg/ml | 78.00± 2.00 |
| 20 mg/ml | 95.000 ± 1.00 |
| Positive Control | 100 ±  0.00 |
| L.S. D | 2.203 |
Note: Mean ± standard deviation
Table 2: Antifungal activity of the crude Alkaloid compounds extracted from seeds of (Carthamus tinctorius L.) against Aspergillus species isolated from stored medicinal plants seeds.
| Concentrations (mg/ml) | Flavonoid compounds |
|---|---|
| Inhibition percentage % | |
| Negative Control | 0 ± 0.00 |
| 5 mg/ml | 71.000 ± 1.000 |
| 10 mg/ml | 88.166± 0.763 |
| 20 mg/ml | 91.000 ± 1.000 |
| Positive Control | 100 ± 0.00 |
| L.S. D | 0.767 |
Note: Mean ± standard deviation.
Table 3: Antifungal activity of the crude Flavonoid compounds extracted from seeds of (Carthamus tinctorius L.) against Aspergillus species isolated from stored medicinal plants seeds.
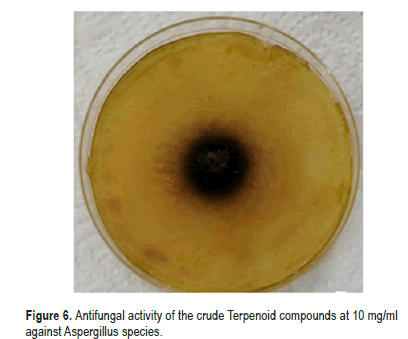
In the same context, the crude terpenoid compounds showed significant activity at three concentrations (5,10 and 20 mg/ml) compared with negative control against Aspergillus species isolated from stored medicinal plants seeds (Table 4). The highest percentage of inhibition (96%) was recorded at 20 mg/ml and (84%) was recorded at 10 mg/ml (Figure 6). While the highest percentage of inhibition in the crude alkaloid compounds was reached up to (95%) at 20 mg/ ml concentration and the highest percentage of inhibition in the crude flavonoid compounds was reached up to (91%) at 20 mg/ml concentration.
| Concentrations (mg/ml) | Terpenoid compounds |
|---|---|
| Inhibition percentage % | |
| Negative Control | 0 ± 0.00 |
| 5 mg/ml | 72.000 ± 2.000 |
| 10 mg/ml | 84.000 ± 1.000 |
| 20 mg/ml | 96.000 ± 1.000 |
| Positive Control | 100 ± 0.00 |
| L.S.D | 1.992 |
Note: Mean ± standard deviation
Table 4: Antifungal activity of the crude Terpenoid compounds extracted from seeds of (Carthamus tinctorius L.) against Aspergillus species isolated from stored medicinal plants seeds.
Discussion
The present study was proved that, the secondary metabolites include alkaloids, flavonoids, and terpenoids extracted from the seeds of (Carthamus tinctorius L.) have powerful antifungal activity against Aspergillus species isolated from stored medicinal plants seeds. The plant kingdom provided and is still providing endless sources of medicinal plants of various uses for example, Bioactive compounds such as phenolic, terpenoids, and alkaloids extracted from several medicinal plants like Lactuca serriola L., Lepidium sativum L., Myrtus Communis L., Cassia senna L., Ricinus communis L., Cassia didymobotrya (Fresenius) Irwin & Barneby, Melia azedarachL., Dianthuscaryophyllus L., and Salvia hispanica L. have antibacterial efficacy against different pathogenic microorganisms. [20-28]. Hussein used primitive plant like Chlorella vulgaris as antibacterial [29]. Kamal used Hibiscus sabdarifa extracts against member of Enterobacteriaceae microorganisms [30]. Kamal used leaves of Ficus caricaLinn against pathogenic bacteria [31]. AL-Masoodi used Curcumalonga L. and Boswellia carteri Birdwood against Fusarium species isolated from maize seeds [32]. The antifungal activity of the carthamin natural pigment of safflower was more active against Candida albicans than precarthamin [33]. The chemical groups isolated from Carthamus tinctorius were included, oils, proteins, minerals, phenolics, flavonoids, alkaloids, lignans, carboxylic acids, steroids, polysaccharides, quinochalcone C-glycosides and quinone- containing chalcones [34]. Safflower oil extracted from seeds exhibited a significant antifungal activity against Candida parapsilosis, Candida sake, and three fungal species aspergillus niger, Penicillium digitatum, and Fusarium oxysporum [35]. On the other hands, the mode of the antifungal action of the Alkaloids is usually pleiotropic, where protein synthesis is inhibited, and the fungal DNA is intercalated or by boosting the development of fungi inhibitors [36]. Terpenoids reduced the mitochondrial content, thus modified the level of reactive oxygen species (ROS) and ATP generation. It is also reported that triterpenoid possesses more potent antifungal activity as compared to the tetraterpenoid [37]. Terpenoids and flavonoids make their effects by disruption of microbial membranes [38]. Medicinal plant possessed antifungal effects by many mechanisms, they caused membrane disturbance resulting in the loss of membrane integrity, inhibited DNA transcription and reduced the cell populations, inhibited the activity of fungal antioxidant enzymes and inhibited fungal biofilm formation [39,40].
Conclusion
Antifungal activity of Carthamus tinctorius L. might be belonging to secondary metabolites like alkaloids, flavonoids, and terpenoids and their effect in proteins and DNA synthesis and disruption in membranes permeability or disturbance in metabolic activity. Alkaloids, flavonoids, and terpenoids extracted from the seeds of (Carthamus tinctorius L.) have powerful antifungal activity against Aspergillus species. Carthamus tinctorius L. The data collected from the study revealed that, the crude alkaloids, flavonoids, and terpenoids compounds extract from seeds of (Carthamus tinctorius L.) showed significant reduction at P ≤ 0.05 in the growth of Aspergillus species especially at 20 mg/ml compared with negative control. Finally, it can be concluded that Carthamus tinctorius L. is most effective in controlling Aspergillus species, especially terpenoids compounds.
References
- Dehariya, Rashmi, and Ashwini Kumar Dixit. "A Review on Potential Pharmacological Uses of Carthamus Tinctorius L." World J Pharm Sci 3 (2015): 1741-1746.
- Shirwaikar, Annie, Saleemulla Khan, Yogesh H. Kamariya and Bharatkumar D. Patel, et al. "Medicinal Plants for the Management of Post-Menopausal Osteoporosis: A Review." The Open Bone Journal 2 (2010).
- Bae, Chun-Sik, Chang-Hyun Park, Hyung-Jin Cho and Hye-Jeong Han, et al. "Therapeutic Effects of Safflower (Carthamus Tinctorius L.) Seed Powder on Osteoporosis." Applied Microscopy 32 (2002): 285-290.
- Zhou, Fei-Ran, Ming-Bo Zhao, and Peng-Fei Tu. "Simultaneous Determination of Four Nucleosides in Carthamus Tinctorius L. and Safflower Injection Using High-Performance Liquid Chromatography." Journal of Chinese Pharmaceutical Sciences 18 (2009): 326.
- Kim, Sung-Kyu, Hyun-Jung Kim, Byoung-Hee Jeong, Jae-Young Cha, and Young-Su Cho. "Properties of the chemical composition of safflower (Carthamus tinctorius L.)." Korean J. Life Sci 5 (2000): 431-435.
- Chopra, Arvind, and Vijay V. Doiphode. "Ayurvedic Medicine. Core Concept, Therapeutic Principles, and Current Relevance." Med Clin North Am 86 (2002): 75-89.
- Maurya, Rakesh, Geetu Singh, and Prem P. Yadav. "Antiosteoporotic Agents from Natural Sources." Studies in Natural Products Chemistry 35 (2008): 517-548.
- Al-Snafi, Ali Esmail. â??Iraqi Medicinal Plants with Antifungal Effect-A Review.â? IOSR Journal of Pharmacy 9 (2019): 16-56.
- Ghazanfar, Shahina, John R. Edmondson and D. J. Nicholas Hind. Flora of Iraq Volume 6. Chicago: The University of Chicago Press, USA, (2019).
- Harborne, Jeffrey, Helga Marby, and T. J Marby. Physiology and Function of Flavonoids. New York: Academic Press, USA, (1975).
- Harborne, Jeffrey. Phytochemical Methods. London: Chapman and Hall, UK, (1973).
- Boham, Bruce, and R. Kocipai-Abyazan. "Flavonoids and Condensed Tannins from Leaves of Hawaiian Vaccinium vaticulatum and V. calycinium." Pacific Sci 48 (1974): 458-463.
- Harborne, Jeffrey. Phytochemical Methods. London: Chapman and Hall, UK, (1984).
- Al-Jassani, Mohammad J. "Tropaeolum Majus Leaves Extract as an Antifungal, Antiaflatoxigenic and Antiaflatoxin Agent." Journal of Global Pharma Technology 12 (2017): 328-333.
- Pitt, John I., and Ailsa Diane Hocking. Fungi and food spoilage. Vol. 519. New York: Springer, USA, (2009).
- Barnett, H.L. and Hunter, B.B. Illustrated Genera of Imperfect Fungi. 3rd Edition. Minneapolis: Burgess Publishing Co., USA, (1972).
- Domsch, Klaus Heinz, Walter Gams and Traute-Heidi Anderson. Compendium of Soil Fungi. London: Academic Press, UK, (1980).
- Gupta, S., and A. B. Banerjee. "A Rapid Method of Screening Antifungal Antibiotic Producing Plants." Indian J Exp Biol 8 (1970): 148-149.
- Singh, Jaspal, and N. N. Tripathi. "Inhibition of Storage Fungi of Blackgram (Vigna Mungo L.) by Some Essential Oils." Flavour and Fragrance Journal 14 (1999): 1-4.
- Al-Marzoqi, Ali Hussein, Hussein J. Hussein and Nebras M. Sahi Al-Khafaji. "Antibacterial Activity of the Crude Phenolic, Alkaloid and Terpenoid Compounds Extracts of Lactuca serriola L. on Human Pathogenic Bacteria." Chemistry and Materials Research 7 (2015): 8-10.
- Al-Marzoqi, Ali Hussein,Nebras M. Sahi Al-Khafaji and Hussein J. Hussein. â??In vitro Antibacterial Activity Assessment of the crude Phenolic, Alkaloid and Terpenoid compounds extracts of Lepidium sativum L. on Human Pathogenic Bacteria.â? International Journal of ChemTech Research 9 (2016): 529-532.
- Hussein, Hussein J., Nebras M. Sahi Al-Khafaji, Ahmed Habeeb Al-Mamoori and Wurood Alwan Kadhim Juaifer, et al. "Antimicrobial Effect of the Crude Phenolic, Alkaloid and Terpenoid Compounds Extracts of Myrtus Communis L. against Human Gram-Negative Pathogenic Bacteria." J Global Pharma Tech 9 (2017): 130-133.
- Hussein, HusseinJ, Nebras M. Sahi Al-Khafaji, Amed Habeeb Al-Mamoori, and Ali Hussein Al-Marzoqi. "Evaluation of In Vitro Antibacterial Properties of the Crude Phenolic, Alkaloid and Terpenoid Extracts of Cassia Senna L. against Human Gram-Negative Pathogenic Bacteria." Plant Archives 18 (2018): 354-356.
- Hussein, Hussein J., Ashwak Falih Kaizal, Nebras M. Sahi Al-Khafaji and Zaman Faris Sadiq, et al. "Evaluation of Antibacterial Potential of the Crude Phenolic, Alkaloid and Terpenoid Extracts of Ricinus Communis L. against Gram-Negative Pathogenic Bacteria." J Global Pharma Tech 10 (2018): 384-388.
- Hussein, Hussein J., Nebras Mohammed Sahi, Ali Malik Saad and Huda Jasim Altameme. "The Antibacterial Effect of Bioactive Compounds Extracted from Cassia Didymobotrya (Fresenius) Irwin & Barneby Against Some Pathogenic Bacteria." Annals of Tropical Medicine and Public Health 22 (2019).
- Hussein, Hussein J., and Ali H. Al-Marzoqi. "The Antibacterial Efficacy of the Secondary Metabolites Extracted from (Melia Azedarach L.) Leaves against Pathogenic Microorganisms Isolated from Burns and Gingivitis Infections." EurAsian J Biosci 14 (2020): 561-565.
- Kamil, Shireen S., Hussein J. Hussein, and A. H. Al-Marzoqi. "Evolution of Antibacterial Efficacy of Dianthus Caryophyllus L. Extracts against Some Hospitals Pathogenic Bacteria." Int J Pharma Res 12 (2020): 1274-1279.
- Hussein, Hussein J., Sabreen A. Kamal, and Nebras Mohammed Sahi. "Antibacterial Efficacy of the Seed Extract of Saliva Hispanica L. against Pathogenic Bacteria Isolated from Diarrhea Cases." Biochemical and Cellular Archives (supp 2) 20 (2020): 3491-3494.
- Hussein, Hussein J., Sura S. Naji, and Nebras M. Sahi Al-Khafaji. "Antibacterial Properties of the Chlorella Vulgaris Isolated from Polluted Water in Iraq." J Pharma Sci Res 10 (2018): 2457-2460.
- Kamal, Sabreen, Hussein J. Hussein, and Zainab A. Tolaifeh. "Antibacterial Potential of Hibiscus Sabdarifa L. against Some Enterobacteriaceae: In Vitro." Biochemical and Cellular Archives 19 (2019): 4291-4294.
- Kamal, Sabreen, Hawraa Wahab Al-Kaim, and Hussein J. Hussein. "Antibacterial Activity of Phytochemical Compounds Extracted from Ficus Carica Linn. Leaves against Human Pathogenic Bacteria." EurAsian Journal of BioSciences 14 (2020): 2293-2298.
- AL-Masoodi, H., Hussein J. Hussein and Abeer Fauzi Al-Rubaye. "Antifungal Activity of the Two Medicinal Plants (Curcuma longa L. and Boswellia Carteri Birdwood) Against Fusarium Species Isolated from Maize Seeds." Int J Pharm Res 12 (2020): 408-414.
- Salem, Nidhal, Kamel Msaada, Salem Elkahoui and Giuseppe Mangano, et al. "Evaluation of Antibacterial, Antifungal, and Antioxidant Activities of Safflower Natural Dyes during Flowering." BioMed Res Int 2014 (2014).
- Chang, Hung-ta, Han HX, Tu PF. â??Chemical Constituents and Pharmacological Effects of Safflower.â? Xiandai Yaowu Yu Linchuang 14 (1999): 201-203.
- Khémiri, Ikram, Badiaa Essghaier, Najla Sadfi-Zouaoui, and Lotfi Bitri. "Antioxidant and Antimicrobial Potentials of Seed Oil from Carthamus Tinctorius L. in the Management of Skin Injuries." Oxidative Medicine and Cellular Longevity 2020 (2020).
- Arif, Tasleem, J. D. Bhosale, Naresh Kumar and T. K. Mandal, et al. "Natural Productsâ??Antifungal Agents Derived from Plants." J Asian Nat Prod Res 11 (2009): 621-638.
- Haque, Enamul, S. Irfan, M. Kamil and S. Sheikh, et al. "Terpenoids with Antifungal Activity Trigger Mitochondrial Dysfunction in Saccharomyces Cerevisiae." Microbiology 85 (2016): 436-443.
- Okusa, Philippe, Stevigny Caroline and Pierre Duez. Medicinal Plants: A Tool to Overcome Antibiotic Resistance? Medicinal Plants: Classification, Biosynthesis and Pharmacology. New York: Nova Science Publishers, USA, (2009).
- Evensen, Nikki, and Phyllis C. Braun. "The Effects of Tea Polyphenols on Candida Albicans: Inhibition of Biofilm Formation and Proteasome Inactivation." Can J Microbiol 55 (2009): 1033-1039.
- Wu, Ting, Mengying He, Xixi Zang and Ying Zhou, et al. "A Structureâ??Activity Relationship Study of Flavonoids as Inhibitors of E. coli by Membrane Interaction Effect." Biochim Biophys Acta 1828 (2013): 2751-2756.
Citation: Hussain, Arabia Yaqoub, Hussein J. Hussein and Abeer Fauzi Al-Rubaye. “Antifungal Activity of the Secondary Metabolites Extracted from Carthamus tinctorius L. against Aspergillus Species Isolated from Stored Medicinal Plants Seeds in the Iraqi Markets.” Clin Schizophr Relat Psychoses 15S(2021). Doi:10.3371/CSRP.HAHH.081221.
Copyright: © 2021 Hussain AY, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.